Everything we do at the DCRI is based on collaboration. Our researchers and operational teams work closely with each other, with our colleagues from the larger Duke University and Duke Health, and with our peers and partners around the world. As an independent academic research organization, we are able to challenge conventional approaches and explore innovative ways to accelerate the translation of scientific discovery into better care for patients everywhere.
What We Offer
- Full integration and close collaborations among faculty and operational leaders
- Fit-for-purpose trial design and operations
- Collaborative approach to academic leadership
- Mature North American site investigator networks that can be leveraged for high-quality enrollment
- Streamlined data collection and adverse event reporting
- Focused, risk-based monitoring and predictive modeling
- Emphasis on study drug compliance and complete follow-up
- Shared endpoint adjudication activities
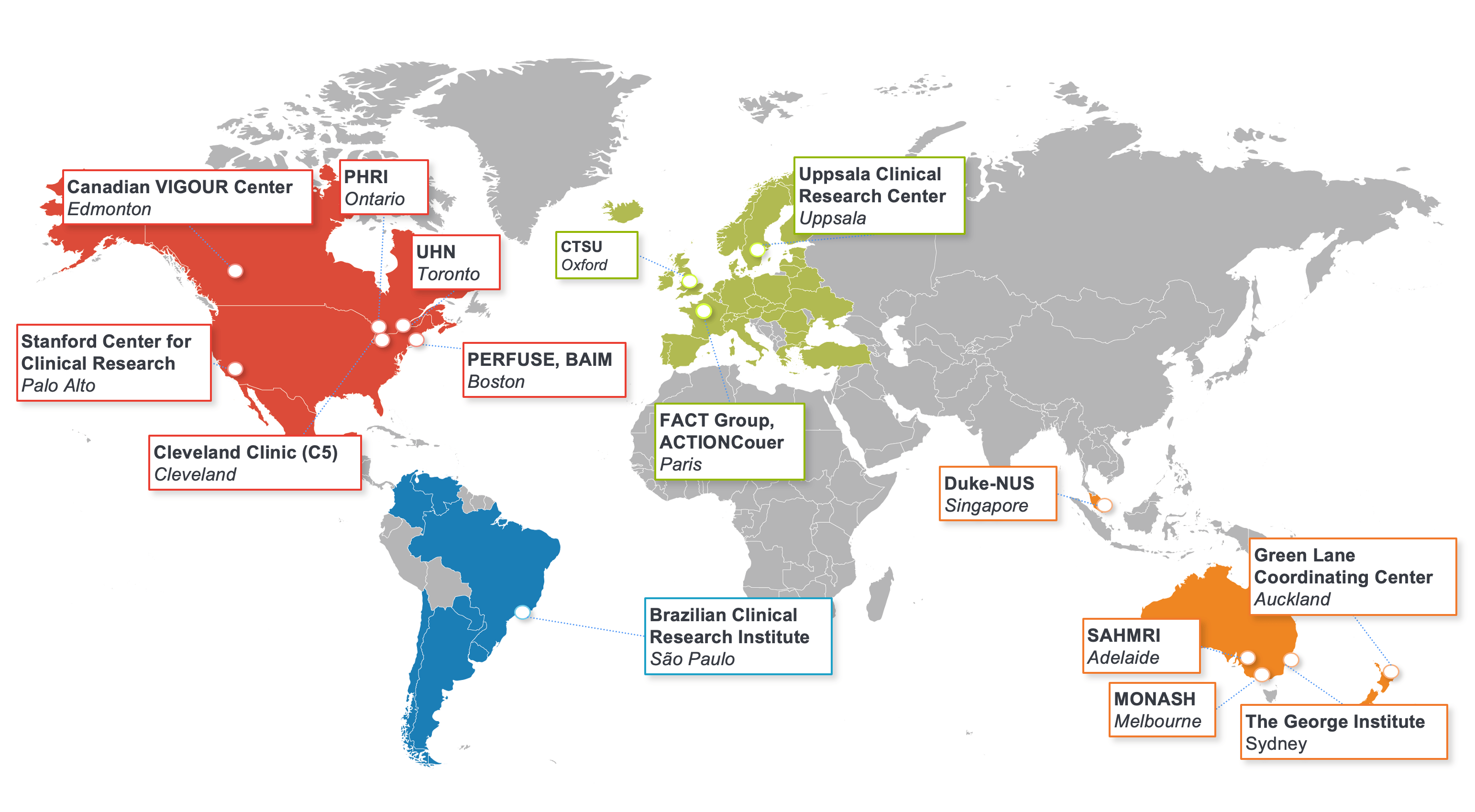
Collaborating to Improve Care
The DCRI pursues a collaborative approach with fellow academic institutions, patient advocacy groups, and public and private organizations throughout the world.
Patients
We are constantly exploring ways to actively engage with and empower patients as full partners in research. The DCRI works closely with patient advocacy groups across a wide variety of therapeutic areas to ensure that patient voices are heard at all stages of the research process. The DCRI Research Together™ program is the latest example of our commitment to engaging patients in meaningful ways.
Investigators/Sites
Together with our extensive networks of site community partners, we’re addressing research challenges that will shape the future of medical care throughout the world. The DCRI's regional, national, and global collaborations make possible immediate access to physicians, nurses, and other healthcare personnel working at investigational sites around the world.
Professional Societies
Our close engagement with professional societies allows us to make meaningful contributions to developing and promoting new and updated clinical practice guidelines—an essential part of ensuring high-quality, evidence-based care. One recent example includes a joint initiative of the American Heart Association and the American Stroke Association called “Get With The Guidelines” (GWTG). This a hospital-based quality-improvement program is designed to improve the care and outcomes of patients with heart disease or stroke.
Government

From its beginning, the DCRI has enjoyed close engagement with governmental entities, including the NIH and FDA. These partnerships have led to innovations in research design and conduct with significant impacts on patient care and public health. The DCRI currently serves as a coordinating center for projects including the NIH Health Care Systems Research Collaboratory and the Pediatric Trials Network.
Industry

We understand the vital role the medical products industry plays at multiple points along the spectrum of therapeutic development and evaluation. We partner with industry experts on many projects, including the SOAR™ (Supporting Open Access for Researchers) initiative. This unique alliance among industry, academia, and government is intended to open clinical research data for the benefit of the broader research community.
Academia
As part of Duke University, working collaboratively with other great institutions of learning around the world comes naturally to us. We are constantly seeking out these partnerships because we know that bringing fresh perspectives and different ways of thinking to any research challenge leads to better and more innovative solutions.
Sharing Knowledge
The DCRI is committed to the idea that clinical research should go beyond just supporting regulatory approval for medical products. We believe that sharing data openly with researchers, physicians, and other stakeholders around the world helps to inform the next research question. To that end, since 1996 DCRI faculty and staff have authored over 16,000 peer-reviewed publications and our research findings have been cited in over 760,000 scientific articles. By contributing to every aspect of research—from basic science to implementation—we are advancing the state of the art in evidence-based care.
Science Culture and Accountability
Duke University is committed to maintaining the highest quality and integrity of all its scientific enterprises. Consistent with this commitment, the DCRI implements mechanisms to guarantee the responsible management and critical review of scientific data.
The DCRI is committed to ensuring that policies and procedures are in place to reflect the highest professional conduct and to promote a culture in which scientific results are critically reviewed and accountability for data integrity is clearly delineated. In addition, Institute policies allow concerns about data integrity to be raised without fear of reprisal and are the foundation by which these concerns are addressed fairly and expeditiously.
To ensure the active participation of all parties in the research mission, from faculty and staff to leadership, the DCRI has created a Science Culture and Accountability Plan.

The Duke Advantage
As part of the Duke University family, the DCRI benefits from the resources of one of the nation's premier research universities and the deep expertise of a nationally ranked health system. Our insights and know-how are drawn both from the arena of clinical trials and from the real world of patient care and community health. Duke's commitment to cross-cutting research also offers unique opportunities for the DCRI to support innovative partnerships and projects— from geospatial mapping to biomedical engineering, to health economics.