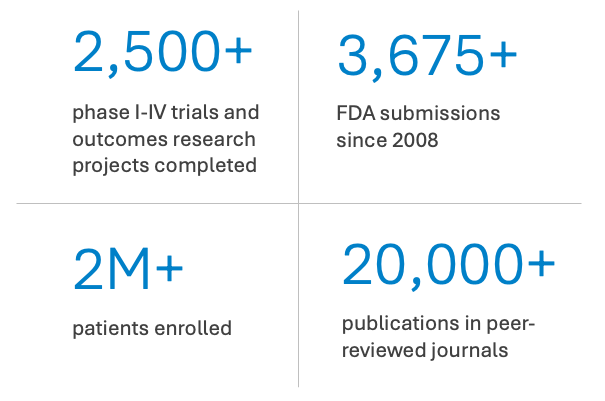
Known for conducting groundbreaking multinational clinical trials, managing major national patient registries, and performing landmark outcomes research, we combine insights from practicing physicians in our world-class health system with unmatched data science expertise. Our end-to-end operations infrastructure ensures that studies and trials of all sizes run effectively, achieve optimal outcomes, and transform healthcare for patients everywhere.
Our Key Clinical Areas
Cardiovascular, Renal & Metabolic | Gastroenterology | Infectious Diseases | Musculoskeletal | Neurosciences | Pediatrics | Respiratory & Immunology
Innovative & Efficient from Development to Commercialization
Integrated services for each trial phase, at every stage
Throughout our 50+ year history, both industry and the government have trusted the DCRI for their most important research across trial phases and sizes. We have the depth and breadth of capabilities to engage with sponsors across the entire research lifecycle of a drug, device, or therapy - from study design to execution to post-approval research, including implementation science.
The DCRI has all the operational services and capabilities of any full-service CRO, including:
- Study Design
- Participant Recruitment and Retention
- Participant Research Operations (Call Center)
- Endpoint Adjudication
- Site Management/Monitoring
- Publication & Medical Writing
- Regulatory Strategy
- Safety
- Protocol Development
- Imaging, Arrhythmia & Hemodynamics Labs
- Data Management
- Biostatistics & Data and Safety Monitoring Board
Reliable Faculty and Staff
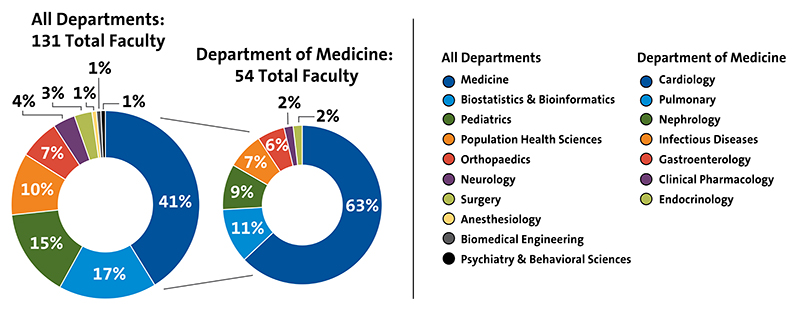
Our team includes over 100 clinical researchers and 800+ operations, data collection, and coordination staff, all working together to create an efficient trial experience. Our faculty leaders stand among the world's foremost authorities in clinical research, bringing deep expertise to the scientific, operational, financial, and regulatory aspects of diverse project designs. The stability of our team is reflected in their impressive tenure, with project leaders averaging 10 years of service. This continuity ensures that the same dedicated professionals who initiate your project will guide it through completion, providing consistent support throughout your research journey.
An Extensive Site Network
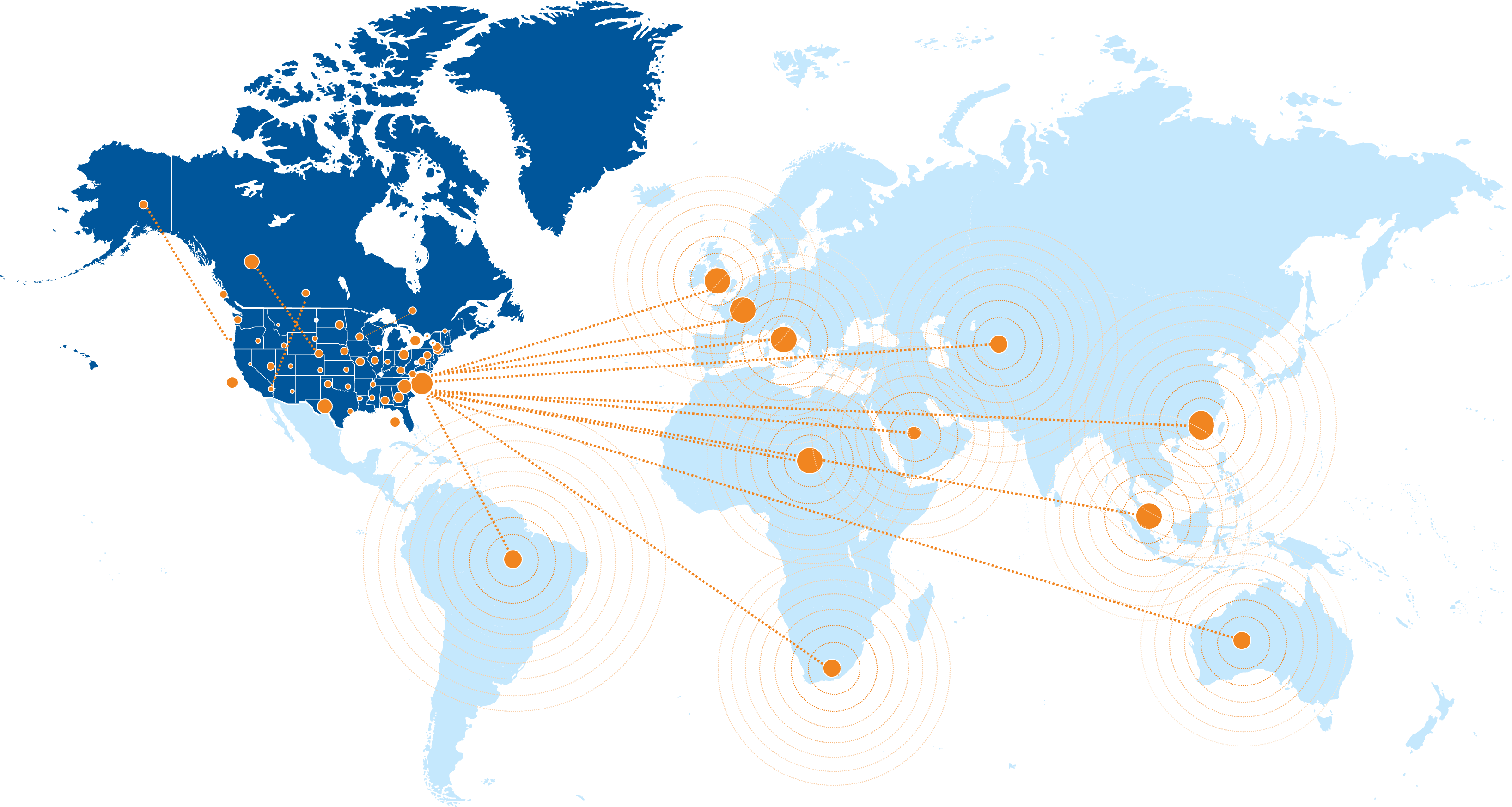
The DCRI is a trusted partner across the U.S., Canada, and globally, with approximately 1,100 research sites throughout North America. Our clinical faculty maintain extensive personal relationships, while our strong connections with academic groups worldwide enable us to identify national leaders and high-performing sites overseas. We partner with CROs around the world for operational support and collaborate with international organizations to conduct global trials to ensure consistent quality.
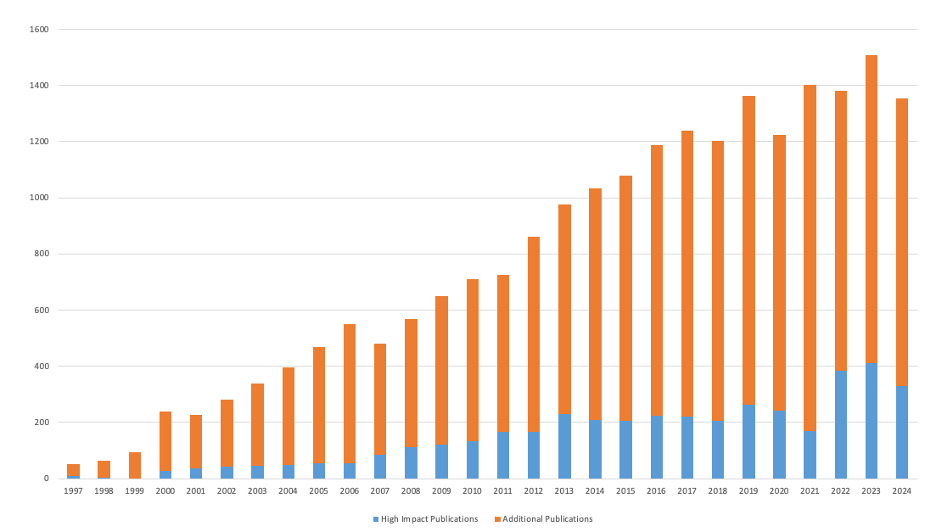
High-Impact Publications
DCRI’s mission is to develop and share knowledge that improves health through innovative clinical research. One of the primary ways in which we do this is through scientific publications. The DCRI has produced over 21,600 publications since 1996.