Research conducted by physicians who treat patients with respiratory diseases every day provides insights that traditional contract research organizations (CROs) cannot offer. Our practicing clinicians connect real-world patient care with innovative clinical trials, ensuring that every study we conduct has exceptional clinical relevance.
Chronic lung diseases affect millions of people around the world, are a leading cause of death, and cause breathlessness, frequent hospitalizations, and impaired quality of life. Despite treatments currently available, many patients continue to experience a decline in respiratory function, increased disability, and premature death.
Conducting the necessary research to develop new treatments for respiratory diseases can be complex and challenging to execute. However, the DCRI Respiratory Medicine team has the experience and clinical expertise to ensure efficient and timely completion of studies. With over 20 years of proven success in respiratory research, we've established trusted partnerships with foundations, federal agencies, and industry sponsors.
Disease Area Expertise
Our team provides expertise across all areas of pulmonary, critical care, and sleep medicine, with particular strength and experience in the following areas:
- Chronic obstructive pulmonary disease (COPD) and emphysema
- Interstitial lung disease (ILD), including progressive pulmonary fibrosis (PPF) and Idiopathic pulmonary fibrosis (IPF)
- Bronchiectasis
- Asthma and airway biology
- Pneumonia
- Acute respiratory distress syndrome (ARDS)
- Rare lung diseases
- Lung transplantation
Innovation in Action
DCRI's Respiratory Medicine team is committed to conducting cutting-edge research to advance treatment options and improve patient outcomes. We combine scientific expertise with innovative methodologies to execute high-quality and efficient clinical trials for our sponsors.
Our portfolio of innovation includes:
- Leading COPD studies that utilize digital inhaler devices while collaborating with a network of community-based research sites, resulting in higher quality data capture and improved patient adherence.
- Leveraging decentralized approaches in IPF studies to ensure comprehensive long-term follow-up for patients, significantly enhancing retention rates and reducing missing data points critical for regulatory submissions.
- Conducting pivotal clinical trials for IPF that have influenced clinical practice, particularly in advancing lysophosphatidic acid antagonism, demonstrating our ability to execute studies with meaningful clinical impact.
- Demonstrating exceptional recruitment of patients with rare lung conditions, including lung transplants, accelerating timelines in traditionally challenging enrollment scenarios.
- Building strategic partnerships with organizations and CROs to enhance capabilities and support our sponsors' goals, providing flexible, scalable solutions tailored to your specific program needs.
Our innovative, effective, and reliable approach helps sponsors navigate the complex landscape of respiratory medicine development with confidence, delivering the rigorous scientific insights and operational excellence needed to advance your respiratory portfolio.
Disease Spotlight
Interstitial Lung Disease
ILD, including IPF and PPF, remains a challenging therapeutic area with significant unmet medical needs. The DCRI Respiratory Medicine team brings proven expertise across all phases of ILD clinical research:
- Registry Leadership: Our IPF- and ILD-PRO registries have enrolled over 2,000 patients across 50 centers, generating real-world evidence on approved therapies, outcomes, and biomarkers for disease progression. Learn more about the registry
- Clinical Trial Execution: Phase II and III trials evaluating novel therapies using both traditional and innovative pragmatic trial designs in collaboration with industry and government partners.
- Data Innovation: Advanced analyses of trial and EHR datasets to define synthetic control strategies that improve clinical trial efficiency for IPF and other ILDs.
- Real-World Evidence: Collaboration with PCORnet networks to characterize real-world ILD populations and assess disease progression risk using electronic health records.
The DCRI Advantage

Clinician-Led Research + Full Service CRO Capabilities
In addition to leveraging the expertise of our practicing physicians and innovative data scientists, the DCRI offers full-service CRO operational capabilities and the unique advantages of being embedded within Duke University's world-class academic medical center infrastructure. With decades of specialized experience in executing complex clinical trials, we are able to reduce study startup timelines compared to traditional CROs. Our proactive operational approach helps us anticipate challenges and deliver effective solutions, ensuring that your trials stay on schedule and within budget.
DCRI’s capabilities include, but are not limited to:
- IRB and regulatory submissions
- Site feasibility, selection, training, and initiation
- Site-, medical-, and safety monitoring
- Full-service electronic data capture and database services
- Data delivery suited for regulatory submissions
- Pharmacovigilance
- Computable phenotypes/EMR-based pre-screening
- Integrated patient recruitment, engagement, and retention solutions
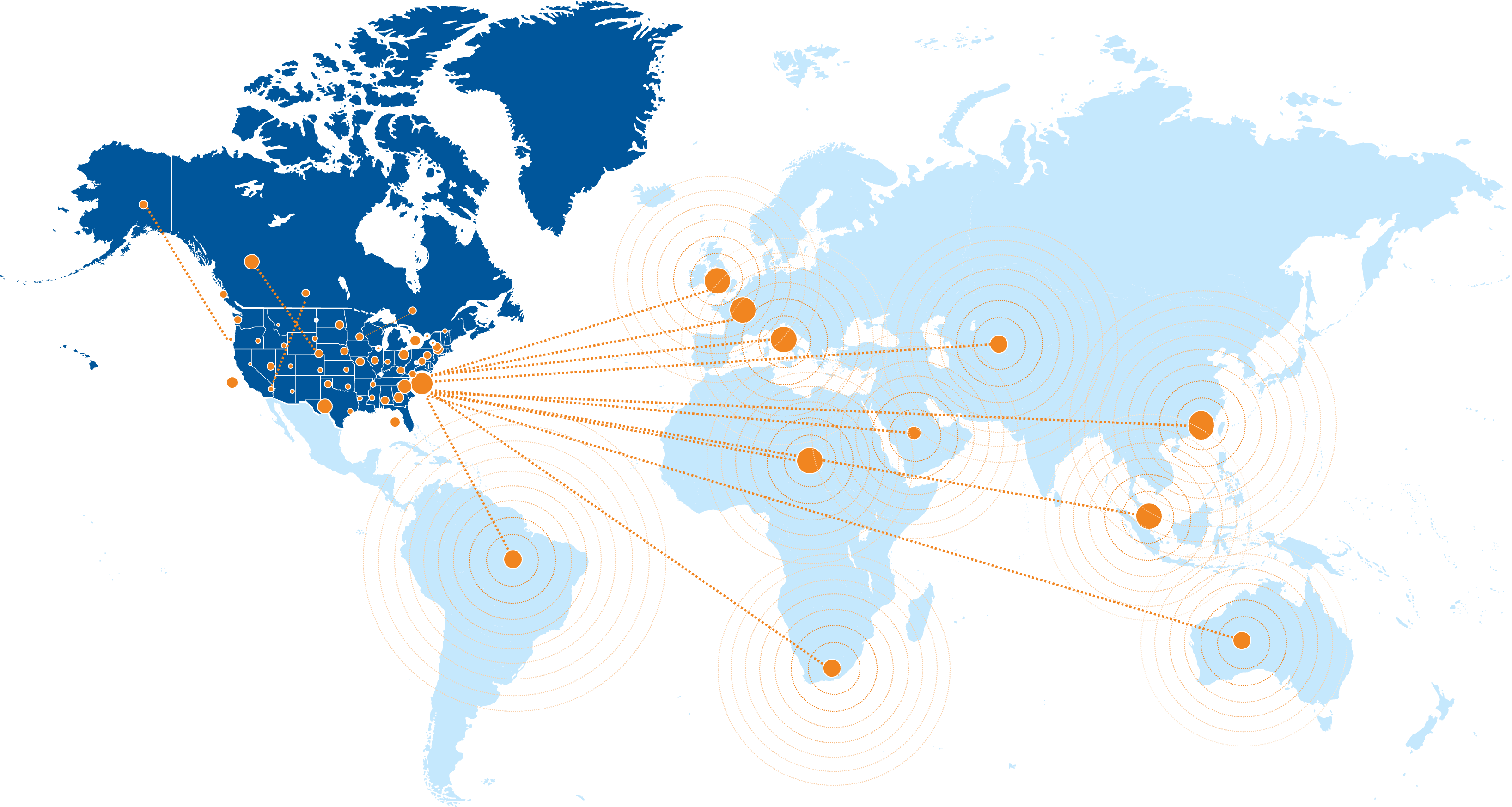
Site Network & Partners
Our trusted network spans over 1,100 research sites across the U.S., Canada, and globally, and includes academic and community-based centers. Through established CRO partnerships, we offer flexible options for conducting studies worldwide, with site selection tailored to specific disease areas and study requirements.
Faculty Leadership
The DCRI Respiratory Medicine faculty has published over 500 peer-reviewed manuscripts in the field of respiratory diseases and serves on the editorial boards of leading respiratory journals. They serve as principal investigators, steering committee members, and key opinion leaders for major industry trials, bringing insider knowledge of regulatory pathways and clinical development strategies to every project. Our faculty, as part of Duke's expansive health system, also draws on the specialized expertise of the broader Department of Pulmonary, Allergy, and Critical Care team. As a result, the DCRI is equipped to provide in-house key opinion leadership for studies covering a wide variety of respiratory conditions.
Ready to Advance Respiratory Medicine Together?
Whether you're planning a first-in-human study or a pivotal regulatory trial, we're ready to discuss how our unique combination of clinical expertise and operational excellence can drive your project forward.





