
Clinical Expertise Meets Research Innovation
As an academic clinical research organization, the DCRI combines the clinical expertise and academic leadership of a premier teaching hospital with the capabilities of a full-service clinical research organization (CRO). Our faculty and operational experts possess the regulatory knowledge, scientific leadership, and collaborative approaches necessary to bring innovative approaches to research challenges, delivering results that advance medicine and improve lives globally.
Our Unique Advantage
Practicing Physicians
Our researchers include practicing physicians from Duke University School of Medicine, representing multiple disciplines, who design trials grounded in the real-world needs of patients. This approach expands the research impact beyond regulatory approval to advance our fundamental understanding of health and disease.
Operational Excellence
Our skilled team of over 600 operations, data collection, and coordination staff implements customized operational strategies, from trial design to execution, to ensure that all studies and trials, regardless of size, are conducted effectively and achieve optimal outcomes.
Robust Engagement Opportunities: The DCRI's extensive U.S. site network, combined with global partnerships, encompasses over 3,500 study sites worldwide, resulting in increased opportunities to engage with key patient populations.
Regulatory & Global Expertise
We possess extensive knowledge of FDA requirements and regulatory pathways, combined with a deep understanding of local, national, and international clinical practice patterns, which accelerates speed-to-market for pharmaceutical and biotech sponsors.
Comprehensive Clinical Trial Capabilities
With decades of specialized experience executing complex clinical trials, we deliver accelerated study startup timelines, exceptional data quality, and regulatory-compliant results that transform healthcare for patients everywhere.
Our Mission
To develop, share, and implement knowledge that improves health around the world through innovative clinical research.
Our Vision
To be the leading academic clinical research organization that:
- Generates world-class evidence to improve health;
- Creates novel methods that accelerate clinical research;
- Shares and implements knowledge widely;
- Develops the next generation; and
- Improves healthcare for all through our research.
Our Values
At the DCRI, our values honor our history and guide our actions and daily decisions. Our values and our mission help us define what it means to be successful—as an organization, as individuals, and as a partner to our sponsors. Together, we are making tomorrow better than today—one patient, one question, one innovation at a time.
INTEGRITY • EXCELLENCE • RESPECT • BELONGING • INNOVATION • TEAMWORK
From Development Through Commercialization Research
Innovation & Expertise
Our clinical operations teams manage trials across all phases and types of research with expertise in data integrity and biostatistics. We leverage our comprehensive infrastructure to deliver consistent, data-driven results. This unmatched expertise enhances study efficiency and reliability by incorporating innovative approaches such as synthetic control arms, master protocols, and artificial intelligence applications that optimize trial design and execution.
Implementation & Real-World Evidence
We don't just conduct clinical trials—we ensure research results improve patient care. Our post-approval research accelerates the adoption of evidence-based interventions, translating insights to improve the uptake of new therapies in real-world healthcare settings. This comprehensive approach from development through commercialization provides pharmaceutical and biotech sponsors with seamless support across the entire product lifecycle.
Our Clinical Research Areas
Across our global network, our physician-scientists provide deep expertise across multiple therapeutic areas critical to pharmaceutical and biotech development, with research conducted by specialists who treat patients with the same conditions every day:
Cardiovascular, Renal & Metabolic | Gastroenterology | Infectious Diseases | Musculoskeletal | Nephrology & Kidney Health | Neurosciences | Pediatrics | Respiratory Medicine
Each therapeutic area is supported by practicing clinicians who understand the unique challenges of developing treatments in their specialty, ensuring that study designs are optimized for regulatory success and that development pathways are clearly understood.
Faculty Leadership Excellence
Our team includes over 100 clinical researchers who stand among the world's foremost authorities in research, bringing deep expertise to the scientific, operational, financial, and regulatory aspects of diverse project designs. Our faculty leaders serve as principal investigators, steering committee members, and key opinion leaders for major industry trials, providing valuable insights into FDA regulatory pathways and clinical development strategies.
DCRI's Commitment to Research for Everyone
The DCRI recognizes that improving health around the world means ensuring that our research reflects real-world populations from a wide range of life experiences, geographic regions, health conditions, and backgrounds. We strive to recognize all of humanity equally, and are committed to improving clinical research and healthcare for all through our studies and initiatives.
Global Site Network & Strategic Partnerships
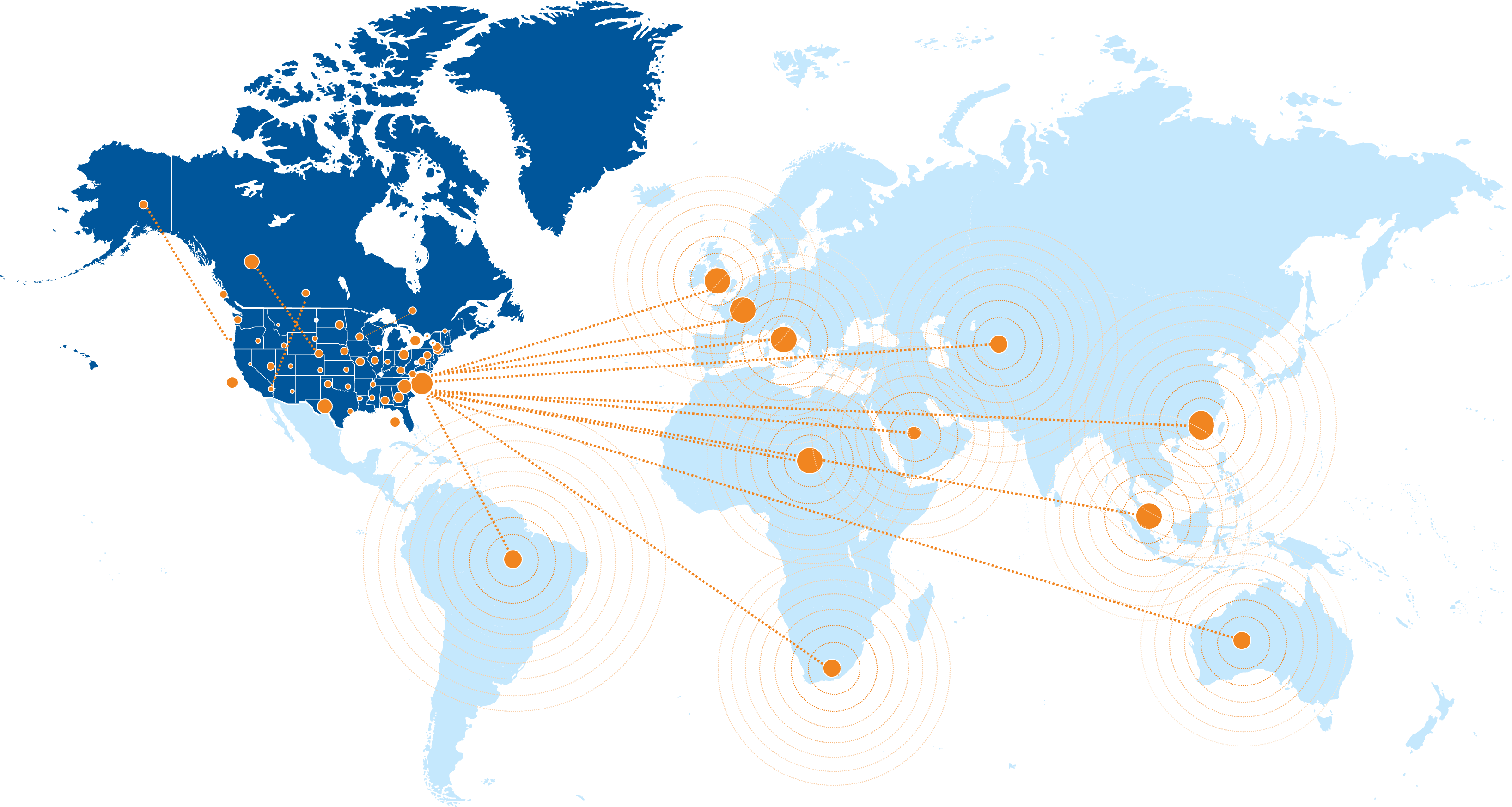
Our trusted network spans over 1,100 research sites across the United States, as well as established partnerships in more than 40 countries. This includes academic medical centers, community-based practices, and specialized research sites, with selection tailored to specific disease areas and study requirements.
Through strategic CRO partnerships, we offer flexible options for conducting studies worldwide while maintaining our high standards for site management, data quality, and regulatory compliance.
As an institute within the larger Duke University School of Medicine and Health System’s collaborative environment, the DCRI also regularly taps into the university’s cutting-edge research and clinical infrastructure. Duke’s internal network includes more than 70 shared service centers in genomics, proteomics, and informatics, as well as over 2,600 basic sciences and clinical faculty.
Industry Recognition
Industry sponsors consistently recognize the DCRI for outstanding partnership, demonstrating our commitment to delivering exceptional results that exceed expectations across all areas of clinical research operations. These awards are based on surveys of biopharmaceutical and medical device companies conducted by Industry Standard Research.
Over the past five years, the DCRI has won CRO Leadership Awards in the categories of Expertise, Quality, Capabilities, Compatibility, and Reliability.
The DCRI has also received individual attribute awards for: Data Quality, Project Timelines, Operational Excellence, and Responsiveness.


Celebrating 30 Years of Shaping Clinical Research...and Beyond
For 30 years, the DCRI has partnered with government, industry, and academic collaborators to generate evidence that changes clinical practice. As we celebrate this milestone throughout 2026, explore the milestones, voices, vision, and expertise we're bringing to the next era of clinical research.





