The Clinical Outcome Assessments for Acute Pain Therapeutics in Infants and young Children (COA-APTIC) study is made possible through the collaboration of the U.S. Food and Drug Administration (FDA), the Duke Clinical Research Institute (DCRI), and the Duke Department of Population Health Sciences.

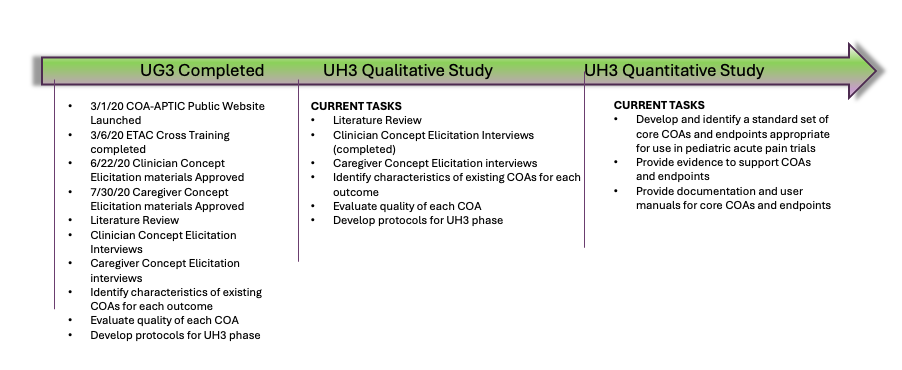
A major barrier to identifying and developing effective treatments for acute pain in infants and young children is the lack of standardized measures and endpoints for use in clinical trials. With a grant from the FDA, the DCRI and the Duke Department of Population Health Sciences are collaborating to identify high-quality clinical outcome assessments (COAs) and endpoints for measuring acute pain in infants and children from birth to 2 years of age.
The project, called Clinical Outcome Assessments for Acute Pain Therapeutics in Infants and young Children (COA-APTIC), aims to develop measures that could be used in clinical trials in the future, which would help open the door for new pain medications to be developed and approved for this age range.
The Center for Health Measurement within the Duke Department of Population Health Sciences will provide its expertise in developing and evaluating measures to be included in this study. Once these outcome assessments are developed, they will be evaluated and validated in a clinical trial setting within the Pediatric Trials Network (PTN).
This program is part of the CDER Pilot Grant Program, which falls under the FDA’s Patient-Focused Drug Development (PFDD) efforts to support the use of meaningful and valid outcomes in regulatory trials.