
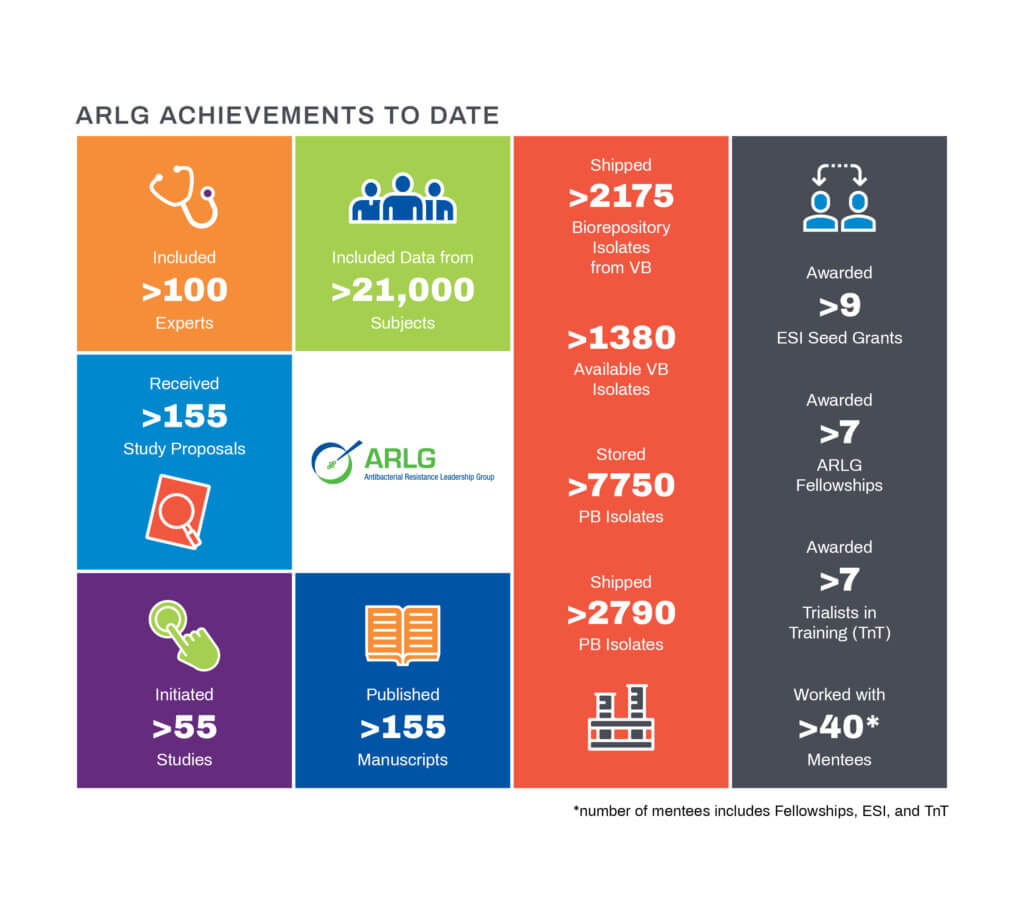
The Antibacterial Resistance Leadership Group (ARLG) involves over 100 experts, generating over 155 papers and initiating 55+ studies with 21,000 participants at 130 sites in 12 countries.
The Antibacterial Resistance Leadership Group (ARLG) conducts clinical research to better understand how to diagnose, treat, and prevent antibacterial resistant infections, which infect 2.8 million people per year in the United States, killing at least 35,000, according to a Centers for Disease Control and Prevention report. ARLG addresses antibacterial resistance through innovative clinical trial design, unique access to clinically well-characterized bacteria, and opportunities for early-stage investigators.[1]
Scientific achievements by the ARLG—which is spearheaded by the DCRI—are described in a paper recently published in Clinical Infectious Diseases.[2] The paper also outlines the agenda and programmatic priorities of ARLG 2.0 in its second funding cycle, which began in December 2019 when the National Institute of Allergy and Infectious Diseases (NIAID) awarded the DCRI up to $102.5M for another seven-year cycle.
“DCRI is uniquely positioned to build and develop networks of this kind, because our infrastructure is ready for use and customizable to the changing needs of this program to mitigate the threat of antibiotic resistance,” said Vance Fowler, MD, ARLG co-principal investigator, member of the DCRI, and professor of medicine at the Duke University School of Medicine. “DCRI combines the ability to solve fundamental scientific problems with extensive operational expertise. This marriage between science and operations is unique.”
The research priorities of ARLG 2.0 are infections caused by antibiotic-resistant Gram-negative and Gram-positive bacteria, and diagnostic tests to optimize use of antibiotics. ARLG 2.0 has an increased emphasis on interventional trials. To support the next generation of researchers, the ARLG offers three mentoring opportunities: the ARLG Fellowship, Early Stage Investigator Seed Grants, and the Trialists in Training Program.
“Our work with ARLG builds on earlier, multidisciplinary experience with other extremely large networks,” said DCRI’s Heather Cross, D.Phil., ARLG Program Leader. “We were able to take best practices from these networks, and build on our exceptional collaborative relationships with academic institutions worldwide. DCRI’s relatively stable workforce and strong team morale are key elements in these relationships.”
The National Institute on Drug Abuse network was the DCRI’s first large-scale network, followed by the Heart Failure Network, and Pediatric Trials Network, all of which paved the way for ARLG 1.0. The DCRI is coordinating center for several other networks, including Environmental influences on Childhood Health Outcomes (ECHO), Trial Innovation Network, and Rapid Acceleration of Diagnostics for Underserved Populations (RADx-UP). Each network has similarities in administrative needs, such as keeping working groups moving forward, and engaging participants in ‘team science’ to achieve progress. Learnings from all of these networks—including identifying essential standard operating procedures (SOPs) and a building a high-performance, large-scale communications platform to provide collaboration spaces—supported the award of ARLG 2.0 via a competitive bid.
“Over time, the DCRI has built ever-stronger relationships with our partners in these networks, earning the role of critical team member, and evolving to achieve extraordinary results,” said DCRI’s Renee Pridgen, MHA, Director, Government Trials and Networks. “The partnership between communications and operations is a major contributor to our success as a coordinating center.”
ARLG 2.0 is investigating the top three bacterial pathogens identified by the World Health Organization as posing a critical risk to human health. These are carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa, and carbapenem-resistant, extended spectrum beta lactamase-producing Enterobacteriaceae (including Klebsiella).[3] Patients with infections caused by Klebsiella pneumoniae and other critical pathogens are being prospectively enrolled at multiple sites in a dozen countries, including China, Singapore, Chile, Columbia, and Argentina. Multiple clonal variations of these pathogens have been identified globally, with significant differences in clinical and molecular epidemiology. The next step, to carry out responsibly designed interventional trials of antibiotics in infections caused by these pathogens, is now underway.
Another important achievement involves ARLG diagnostics efforts. A single set of patient samples was used to develop a ‘gold standard’ for approval of new diagnostics, and this was used to simultaneously gain FDA approval for two diagnostic platforms from separate companies to detect the sexually-transmitted infections chlamydia and gonorrhea.[4] The study was a collaborative, multi-site clinical study of 2,500 patients to evaluate the diagnostic accuracy of multiple commercially available nucleic acid amplification tests for detection of extra-genital chlamydia and gonorrhea.[5]
ARLG has also innovated by developing a new clinical trial endpoint, the desirability of outcome ranking (DOOR). Using DOOR, patients are classified according to overall clinical outcome, taking into account benefits and harms using ordinal categories. This yields a more informative and pragmatic benefit/risk evaluation[6] by incorporating the degree of patient wellness. This approach is currently being evaluated in several common drug-resistant infections in collaboration with the FDA. This has potential for use as a secondary or exploratory endpoint in registration studies, helping companies to differentiate their products based on their specific impacts on the experience of patients with these infections.
Funding for the ARLG is provided by NIAID under award number UM1AI104681.
References
[2] https://academic.oup.com/cid/advance-article-abstract/doi/10.1093/cid/ciab141/6137279
[3] https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed
[4] https://www.fda.gov/news-events/press-announcements/fda-clears-first-diagnostic-tests-extragenital-testing-chlamydia-and-gonorrhea
[5] https://www.fda.gov/news-events/press-announcements/fda-clears-first-diagnostic-tests-extragenital-testing-chlamydia-and-gonorrhea