With $21 million in new funding from the NIAID, the network now includes eight centers in North America and will study adolescent lung transplant recipients as well as adult.
Over 7 years ago, Scott Palmer, MD, MHS, head of DCRI’s Medicine Plus Therapeutic Area, recognized problems in lung transplant care and led an application for funding to address some of these problems. That funding initiated in 2014 and supported the creation of a large multicenter lung transplant research consortium called the Lung Transplant Clinical Trials Network (LT-CTN). Recently, the funding was renewed by the National Institute of Allergy and Infectious Diseases (NIAID), with $21 million in new funding to support a seven-year interventional trial and mechanistic studies.
Palmer will serve as one of three principal investigators for the next chapter of this funding, along with John Belperio, MD, of University of California-Los Angeles (UCLA) and Stuart Sweet, MD, PhD, of Washington University in St. Louis. Palmer recently discussed the new LT-CTN award and plans for the network’s future.
What problem is the Lung Transplant Clinical Trials Network (LT-CTN) trying to solve?
Patients who receive a lung transplant are more likely to experience negative outcomes than patients who receive other commonly transplanted organs. Care for these at-risk patients also varies across different centers.
The LT-CTN convenes top lung transplant centers in North America to come together to find solutions and improve outcomes.
How is the LT-CTN funded?
The NIAID administers the Clinical Trials in Organ Transplantation, or CTOT, program. Previously, CTOT had successfully organized studies in other solid organ transplants, specifically kidney, heart, and liver.
I led the development of a proposal for the initial CTOT funding, which was awarded and began in 2014, creating the LT-CTN, which was the first CTOT-funded program to focus on adult lung transplant.
Which organizations are involved in the LT-CTN?
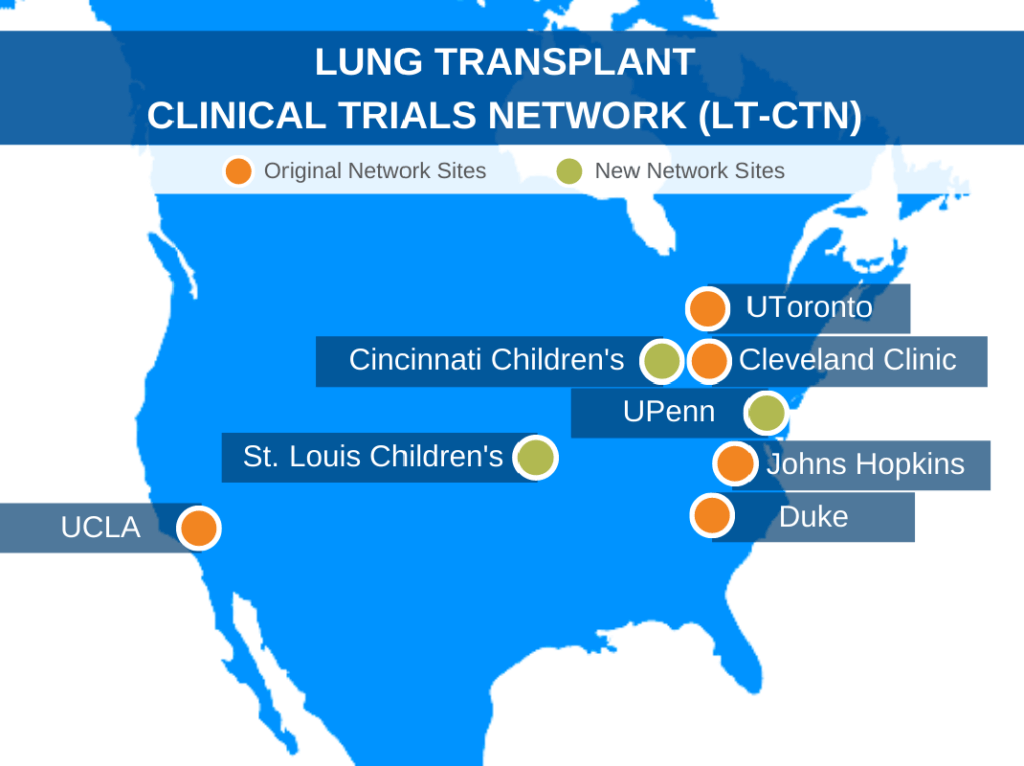
With Duke serving as the lead, the original LT-CTN pulled together four additional institutions across North America with strong backgrounds in lung transplant: Johns Hopkins University, UCLA, Cleveland Clinic, and University of Toronto.
What did the LT-CTN accomplish during its initial CTOT funding cycle?
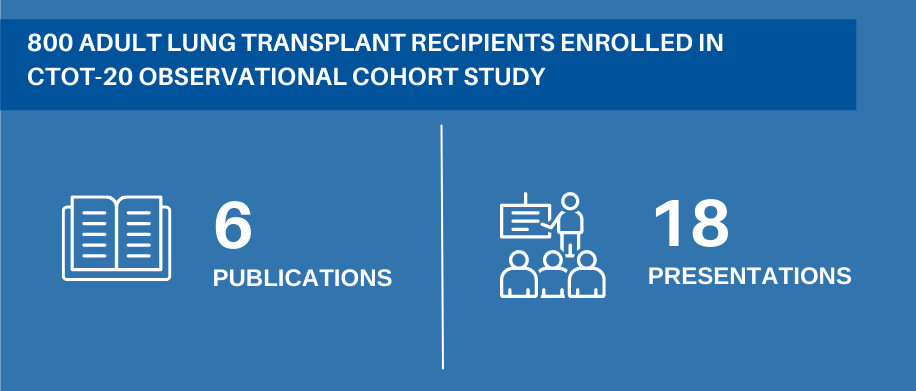
To date, the initial CTOT funding has led to six publications and 18 presentations from the LT-CTN. Perhaps most importantly, the initial CTOT funding helped to create the collaboration and infrastructure that was needed to move the field forward through further multicenter trials. The data that were generated also informed the proposal for the CTOT funding renewal.
The initial work, a prospective observational cohort study called CTOT-20 that enrolled over 800 lung transplant recipients, focused on chronic rejection of a lung transplant, or chronic lung allograft dysfunction (CLAD). At the time, this was the primary problem limiting long term success after lung transplant. CLAD occurs in over half of patients within five years of receiving a lung transplant, and average survival once CLAD occurs is only three years.
The LT-CTN’s overall goals were to better understand the clinical factors and biological mechanisms that contributed to whether a patient would experience CLAD.
Once researchers began to learn more and generate more data, they realized bronchoscopy results and other key findings could help stratify patients by their projected risk for CLAD. These risk stratification methods informed the proposal for a new round of funding, as these methods will be used to deliver a personalized medicine approach and enroll only the highest-risk patients into the next study.
The LT-CTN, which is coordinated by the DCRI, recently received $21 million in CTOT renewal funding.
What efforts will the funding renewal support?
The LT-CTN will use the renewed round of funding, which will be distributed over seven years, to add an additional member, University of Pennsylvania, to the adult lung transplant network. The network will also expand to include children’s centers led by Washington University’s St. Louis Children’s Hospital and Cincinnati Children’s Hospital, enabling the network to expand its focus to also include patients over 12 years old who receive lung transplants.
The LT-CTN now includes eight centers; together, these centers conduct over 600 lung transplants a year.
What is the next study planned?
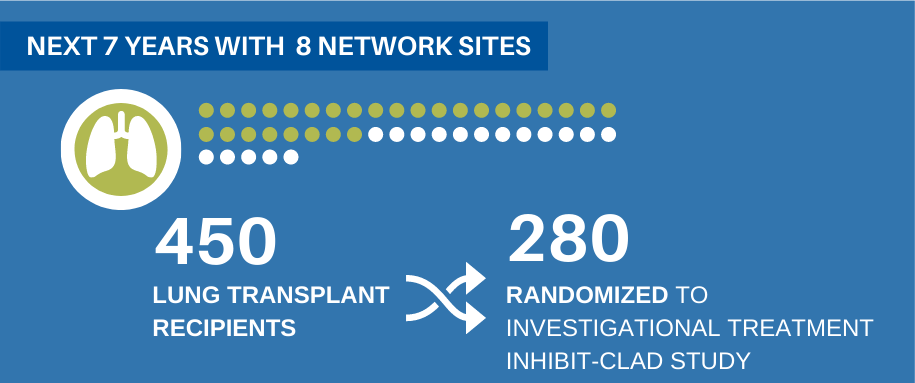
The funding renewal will be used primarily to conduct a randomized clinical trial called INhIBIT-CLAD. This study will enroll 450 adult and pediatric lung transplant recipients across all eight sites, with 280 people randomized to an investigational treatment, which is part of an exciting new class of immune modulating medications. This particular class of drugs has already been approved for treatment of autoimmune diseases, but there has been very little study of their potential usefulness in solid organ transplant.
Biosamples will be collected as part of the study and will be delivered to two core labs at Duke and UCLA, which will study how innate immunity and adaptive immune responses, respectively, are impacted by the drug and how they contribute to CLAD. This process will help us ascertain answers to even more questions, such as who the drug is working in, and what immune processes are still ongoing if it’s not working.
How will the ongoing work of the LT-CTN impact the field of lung transplant and patient care?
Because this condition affects such a large proportion of lung transplant recipients, this study has the opportunity to make a huge impact in the field. Through conducting the INhIBIT-CLAD trial, the LT-CTN is working to expand the paradigm of how immune suppression might help in the prevention of CLAD. Researchers hope to directly apply findings to patient care in order to improve outcomes.
Who are the other DCRI and Duke investigators within the LT-CTN?
DCRI investigators include pulmonologists Laurie Snyder, MD, MHS, and Jamie Todd, MD, MHS, who will oversee the research on biomarkers within the core lab. DCRI pediatrician Jason Lang, MD, MPH, will provide expertise as the LT-CTN expands to also include children.
The success of the project and grant has also relied on key Duke contributors to the grant application, including John Reynolds, MD; Megan Neely, PhD; Courtney Frankel; Jerry Kirchner; Julie Randle; Joanna Downer; and Maile Henson.