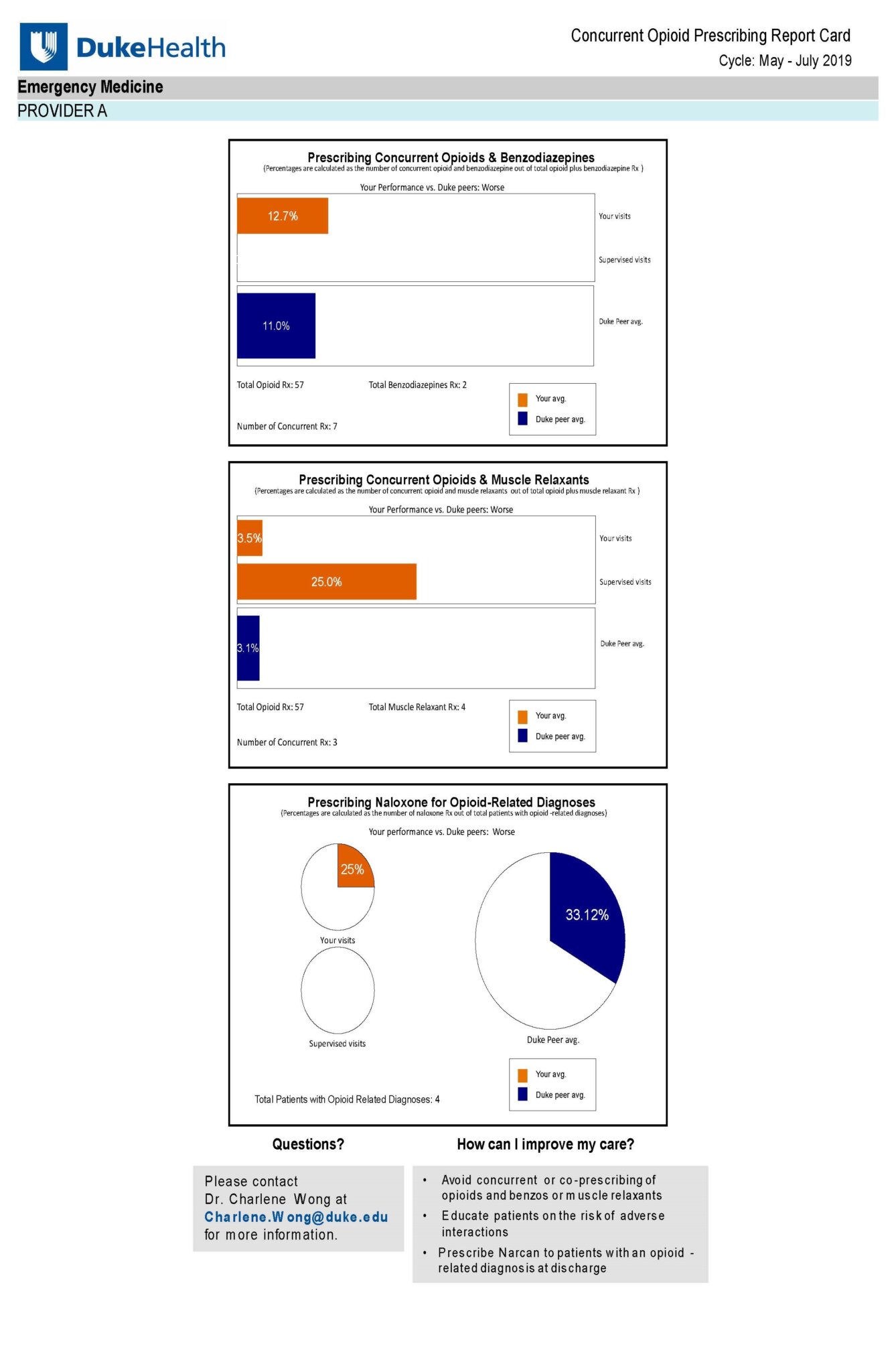
Data from electronic health record (EHR) systems—including on prescribing patterns—are often not available to clinicians in an aggregated or easily digestible format. Researchers in DCRI’s Behavioral Research Intervention Science Center (BRISC) have developed a blueprint to overcome this challenge, providing up-to-date feedback reports to clinicians on their own prescribing patterns compared with those of their peers.
This blueprint automatically creates and sends individualized, graphic reports to clinicians, helping inform clinical practice. A once-monthly frequency was selected for the report, with the aim of avoiding “provider alert fatigue,” which can occur when an individual receives too many electronic alerts.
Based on a study involving an “opioid prescribing nudge,” the blueprint was developed within the Duke University Health System from 2019-2020. The study is titled A Concurrent and Co-Prescribing Opioid Prescribing Nudge: Leveraging Behavioral Economics to Reduce Opioid Harm within Health Systems.
“We looked at how sharing peer prescribing practices might change clinicians’ own prescribing practices, and to understand what their reactions were,” said DCRI’s Charlene Wong, MD, MSHP, co-director of BRISC and an adolescent medicine pediatrician at Duke. “This approach could be applied to yield actionable intelligence from real-world EHR data in any disease or therapeutic area—for example, to improve outcomes in chronic diseases such as diabetes or heart failure.”
Reactions from prescribers involved in testing the blueprint were positive. In response to a survey, one said, “It doesn’t take a lot of time to understand… It’s pretty intuitive in the sense that it’s laid out in a nice bar graph form, so you get a quick overview of where you stand.” Another stated, “I personally like the format this is presented in… I can straightaway see the numbers where I stand, and the bars give me an idea about if I’m doing more than my peers.” A third said, “It's a good reminder in general to think about if you’re prescribing opiates, what other contraindications the patient might have.”
The blueprint sets out a six-step procedure for creating and automating reports on clinician prescribing patterns:
Step 1: Establish Feedback Team
This team—which should include a data aggregator, system programmer, clinician, and coordinator—sets up, monitors, and disseminates feedback reports. Members should agree on a mission and objectives, engage relevant stakeholders, and set quality goals.
Step 2: Define Missions & Goals
The team should identify a clinical problem, such as concurrent prescribing of opioids and benzodiazepines, which increases the risk of opioid-related overdose and death. The team should consider engaging clinician stakeholders to provide feedback on what would be most helpful in their clinical practice.
Step 3: Identify and Engage Clinical Settings
Specific clinical settings that would benefit from receiving feedback reports should be identified, based on specialty or location. A champion with an in-depth understanding of setting should be identified; for example, a clinician who practices in that setting. Any regulatory approval needed to use the EHR data from each clinical setting should be obtained.
Step 4: Develop and Test Feedback Reports
The development and testing of feedback reports should bring together the clinical, analytical, and technical expertise of the team, ensuring that the final reports are both clinically relevant and technically feasible. The team should identify codes needed to create feedback reports from the EHR systems, such as those identifying specific medications or diagnoses.
Step 5: Disseminate Feedback Reports
Setting up an automated process to generate and disseminate feedback reports can dramatically reduce the cost necessary to implement reports. Tests should be carried out to address all functionality of report assimilation and distribution to prove success or illuminate any unforeseen gaps prior to going into production.
Step 6: Monitor and Assess Quality
While the feedback reports may be generated and disseminated automatically, it is important to monitor the report dissemination process to ensure that the reports continue to be accurate and useful to the clinicians receiving them. It can be helpful to set up an automated notification of report generation for the feedback team; create an electronic mailbox dedicated to receiving feedback from clinicians about the report and put the mailbox address on the reports themselves; and consider soliciting verbal feedback from those who receive the reports.
In addition to Wong, the DCRI members of the operational team responsible for this blueprint were: Charles D. Scales, MD, MSHS; William Song; Nisha Datta, MS; Vincent Miller, MMCi; Jennifer Tsapatsaris; and Alfred D’Ottavio. Other Duke members of the operational team included: Stephanie A. Eucker, MD, PhD; Betsy Methven-Dorner, MLS; Maria Grau-Sepulveda, MD, MPH; and Jessica Beliveau, MPH; and the Duke Opioid Collaboratory.