Advancing neurological medicine requires a profound understanding of complex brain pathophysiology, innovative endpoints for assessing target engagement and clinically relevant functional outcomes, as well as the regulatory expertise to navigate the development of first-in-class and first-in-disease-state therapies. The DCRI Neurosciences team combines practicing clinicians who understand the nuances of neurological conditions with proven operational excellence in executing the most challenging studies in the field.
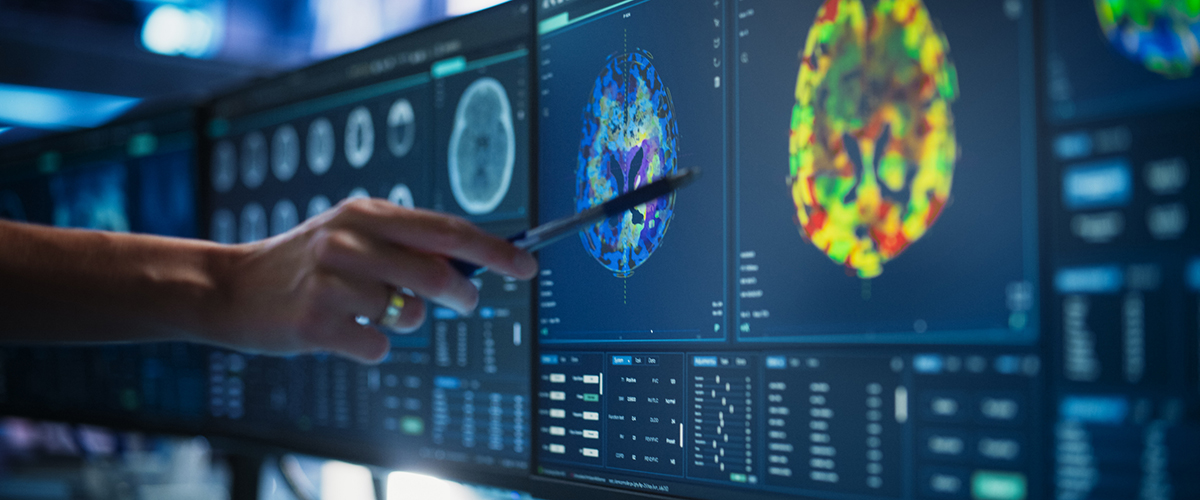
Neurological disorders represent one of medicine's most complex therapeutic frontiers, affecting millions globally while remaining leading contributors to disability and mortality. These conditions have a severe impact on cognition, motor function, and overall quality of life. Even with existing therapeutic options, countless patients face relentless disease progression, mounting disabilities, and substantial gaps in available care.
Developing innovative neurological therapies requires specialized research capabilities beyond standard clinical trial execution. The DCRI Neurosciences team combines experienced clinicians with a robust operational infrastructure. Our deep understanding of neurological pathophysiology and track record in managing complex studies make us the ideal partner for addressing the challenges of neurological drug development and therapies.
Disease Area Expertise
Our team provides expertise across all areas of neurology, neurosurgery, and neuropsychology, with particular strength and experience in the following areas:
Stroke and Cerebrovascular Disease
- Ischemic stroke, intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH)
- Acute stroke intervention and neuroprotection
- Stroke prevention and secondary prevention
- Chronic stroke disability treatments
Neurodegenerative Diseases
- Alzheimer's disease and related dementia syndromes
- Parkinson's disease and movement disorders
- Cognitive aging and mild cognitive impairment (MCI)
Epilepsy and Seizure Disorders
- Status epilepticus and refractory seizures
- Non-convulsive seizures
- Novel electroencephalogram (EEG)-based endpoints
Traumatic Brain and Spinal Cord Injury
- Traumatic brain injury (TBI) and coma
- Spinal cord injury and subdural hematoma
- Neuroprotection and recovery
Neuromuscular and Rare Neurological Diseases
- Multiple sclerosis and demyelinating diseases
- Myasthenia gravis
- Neuromuscular disorders
Neuroimaging and Electrophysiology
- Advanced neuroimaging analysis
- Clinical neurophysiology and EEG adjudication
- Digital biomarkers and assessment tools
- Sleep medicine
Innovation in Action
Breakthrough neurological therapies require research partners who understand both the scientific complexity and operational demands of this challenging field. The DCRI Neurosciences team specializes in transforming ambitious therapeutic concepts into innovative, effective, and reliable clinical programs through our unique blend of clinical insight and research excellence.
Our portfolio of innovation includes:
- First-in-class treatments: Leading the first-ever Phase II study for intracerebral hemorrhage (CATCH trial), demonstrating our ability to navigate high-risk, high-reward therapeutic areas with compelling unmet medical needs.
- Revolutionary EEG endpoints: Pioneering the use of EEG as a clinical endpoint in the TRENdS study, establishing standardized definitions and quality assurance processes that have become the foundation for larger global trials like RAISE I/II.
- Advanced stem cell therapies: Developing novel regulatory strategies for first-in-human stem cell interventions, including intracranial injections for Alzheimer's disease and chronic stroke recovery (GABA-ERON, PISCES studies respectively).
- Digital therapeutics: Achieving FDA clearance for EndeavorRx, the first prescription-only game-based digital therapeutic device approved to improve attention function in children with ADHD.
- Non-pharmacological interventions: Leading a large federally-funded platform (RECOVER-NEURO) to evaluate the use of non-pharmacological interventions, such as transcranial direct current stimulation, adaptive interactive cognitive training, and mindfulness training to mitigate brain fog and cognitive symptoms in patients suffering from long COVID.
- Biomarker-driven trials: Proving therapeutic effect among patients with validated Alzheimer's diagnosis using biomarkers in the Annovis buntanetap study, completed ahead of schedule with effective site identification and rapid enrollment.
The DCRI Advantage

Clinician-Led Research + Full Service CRO Capabilities
In addition to leveraging the expertise of our practicing physicians and innovative data scientists, the DCRI offers full-service clinical research organization (CRO) operational capabilities and the unique advantages of being embedded within Duke University's world-class academic medical center infrastructure. With decades of specialized experience in executing complex clinical trials, we are able to reduce study startup timelines compared to traditional CROs. Our proactive operational approach helps us anticipate challenges and deliver effective solutions, ensuring that your trials stay on schedule and within budget.
Specialized Neurosciences Capabilities
Innovative Clinical Neurophysiology
- Deep expertise in the collection, review, and adjudication of electrophysiology
- Established methods for EEG measures and endpoints
- Standardized training for sites to ensure proper patient selection and interpretation
- Quality assurance processes that minimize variability across sites
Complex Endpoint Management
- Delivering measurable outcomes where outcomes are hard to define
- Integrating biomarkers and their science into trial designs
- Novel assessment tools beyond traditional scales
- Real-time collaboration between adjudicators and study teams
Regulatory Excellence
- First-in-class regulatory strategies for novel interventions
- De Novo premarket review pathway expertise for digital therapeutics
- Investigational new drug (IND) applications for complex biologics and stem cell therapies
- Pre-IND meetings and orphan drug designations
Site Network & Partners
Our trusted network spans over 1,100 research sites across the U.S., Canada, and globally, and includes academic and community-based centers. Through established CRO partnerships, we offer flexible options for conducting studies worldwide, with site selection tailored to specific disease areas and study requirements.
Duke University’s health system is just one of the many sites in our extensive global network. The DCRI Neurosciences team integrates the clinics, resources, and services of this robust ecosystem of expertise, including precision genomics, AI health, population health sciences, and health policy.
Faculty Leadership
The DCRI Neurosciences faculty represents one of the most comprehensive and experienced teams in clinical neuroscience research. Our faculty serve as principal investigators, steering committee members, and key opinion leaders for major industry trials, bringing insider knowledge of regulatory pathways and clinical development strategies to every project. They are recognized leaders in the neuroscience community, serving on editorial boards of leading journals including the Journal of the American Medical Association, New England Journal of Medicine, Stroke, Annals of Neurology, and Clinical Trials.
Ready to Advance Neurosciences Together?
Whether you're developing novel endpoints for complex neurological conditions or conducting a pivotal regulatory trial, we're ready to discuss how our unique combination of clinical expertise and operational excellence can drive your project forward.






