Responding Rapidly to a Pandemic
As the COVID-19 pandemic quickly became the most pressing health issue around the globe, the DCRI worked to rapidly create research programs to learn more about the novel disease. The DCRI’s work in this area has been supported by a number of funders, from the Patient-Centered Outcomes Research Institute (PCORI) to the National Institutes of Health (NIH) to Pfizer.
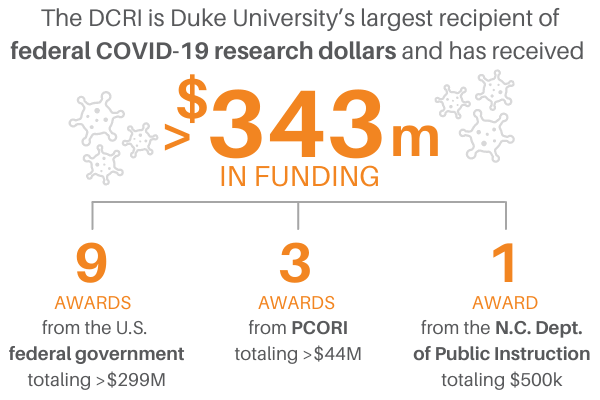
Federally-Supported Programs
Advancing New Treatments
The DCRI plays an essential role in two master protocols that are part of the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program, which is administered by the NIH. The DCRI serves as:
- The U.S. coordinating center for ACTIV-1, which studies multiple immune modulators that may prevent the overactive immune response that occurs in some severe COVID-19 cases.
- The coordinating center for ACTIV-6, a platform trial designed to understand whether already-approved drugs for other indications could be effective in treating COVID-19 at home.

Expanding Access to Testing
The DCRI is partnering with the UNC Center for Health Equity Research and Community-Campus Partnerships for Health to co-lead Rapid Acceleration of Diagnostics for Underserved Populations (RADx-UP). The program, which aims to expand access to COVID-19 testing, is supported by the NIH.
Studying Community Transmission
RADx-UP also supports Say Yes! COVID Test, a program that is testing whether making self-administered COVID-19 tests available in specific communities helps to reduce community transmission. Say Yes! COVID Test is supported by the NIH.
Distributing Free Tests in Underserved Communities
You & Me COVID-Free provided free COVID-19 tests in underserved communities and is evaluating whether they are an effective way to reduce community transmission when used prior to gathering with others.
Highlights

REDUCING TRANSMISSION IN SCHOOLS
DCRI pediatricians lead the ABC Science Collaborative, which found that last fall, school districts that required masking within schools saw lower rates of COVID-19 transmission compared to those with optional masking policies.

STUDYING VACCINE SAFETY
The DCRI and Verily Life Sciences are partnering to lead HERO-TOGETHER, a study funded by Pfizer that will follow vaccinated healthcare worker for two years to better understand their experiences after receiving COVID-19 vaccines.
GENERATING EVIDENCE ACROSS THE GLOBE
DCRI’s Renato Lopes, MD, PhD, worked with colleagues in Brazil to conduct a series of trials tackling different questions related to COVID-19, such as whether it is safe to continue ACEI/ARB therapy in patients with COVID-19.
Making Rapid Research a Reality
Like the rest of the world, the DCRI was forced to respond rapidly to the COVID-19 pandemic, launching its first program, a nationwide community of healthcare workers, in a few short weeks. In the video above, watch as DCRI Executive Director Adrian Hernandez, MD, MHS, speaks with DCRI Program Manager Lauren Cohen, MA, about how this project came to fruition so quickly—and which aspects of rapid start-up can be applied to other pressing public health issues.
Sharing Our Expertise
DCRI researchers are not only leading the way forward in developing knowledge about the COVID-19 pandemic; they’re also sharing that knowledge with the public by speaking with the media.
- A study conducted by the ABC Science Collaborative, a group working on a data-driven approach to safely reopen schools, was cited in many national outlets, including The Washington Post, Forbes, and The Atlantic.
- DCRI Executive Director Adrian Hernandez, MD, MHS, spoke with The Washington Post about clinical trials infrastructure during COVID-19. He also authored a STAT First Opinion piece about the COVID-19 vaccine trials.
- DCRI cardiologist John Alexander, MD, MHS, weighed in on how COVID-19 will impact future clinical trials in a conversation with Scientific American.
Collaborating to Fight COVID-19
DCRI researchers have teamed up with colleagues across the University to conduct impactful projects during the COVID-19 pandemic.
- DCRI’s Manesh Patel, MD, partners with the Duke Institute for Health Innovation as a leader of the Pandemic Response Network, a resource that allows people to sign up for help tracking and managing their symptoms.
- DCRI’s Jessilyn Dunn, PhD, is one of the lead investigators for CovIdentify, a study conducted by Duke’s Pratt School of Engineering to determine whether smartphones and wearables can aid in early detection of COVID-19.
- DCRI’s Scott Palmer, MD, MHS, teamed up with colleagues across Duke University School of Medicine to conduct research to better understand why some people have more severe cases of COVID-19 than others.


