The DCRI has partnered with Bristol-Myers Squibb (BMS) to expand access to clinical trial information from BMS-sponsored studies. Clinical trial information being made available for scientific research through SOAR™ includes protocols, full clinical study reports and de-identified patient-level data and study-level data Phase II-IV interventional clinical trials that completed on or after January 1, 2008. In addition, primary results from these trials must have been published in peer-reviewed journals and the medicines or indications approved in the U.S., EU, and other designated markets. Other criteria may apply, for details please visit Bristol Myers Squibb at www.vivli.org
Data Governance
Each proposal is quickly reviewed by an Independent Review Committee with expertise in biostatistics, research ethics, patient privacy, and the clinical specialty of the research to evaluate the statistical analysis plan, plan to protect patient privacy, dissemination plan and qualification of the investigator(s). The data and analytical tools are provisioned to the investigator through the Vivli Data Sharing Platform.
Data Sharing Metrics
Status Definitions
Under Review: The request/proposal is currently being reviewed internally by a qualified panel of Bristol-Myers Squibb experts. If the proposal is considered within scope, the request will undergo an additional review by the independent review committee (DCRI).
Declined/Out of Scope: the request does not meet the current requirements of the BMS Data Sharing Policy.
Approved: Passed BMS evaluation criteria and the criteria of the Independent Review Committee (DCRI).
Data Shared: Investigator has received access to the requested data, via a secure CTDT portal or the Vivli secure research environment.
Published: Public disclosure of the research results, e.g. publication in a scientific journal, poster at a scientific conference, etc.
Manuscript Submitted: Research is complete, and the resulting manuscript has been reviewed by BMS and submitted for publication to a scientific journal.
Withdrawn/Lost to Follow-Up: Researcher either has withdrawn the research proposal, at any stage, or there is no reply regarding follow-up clarifications.
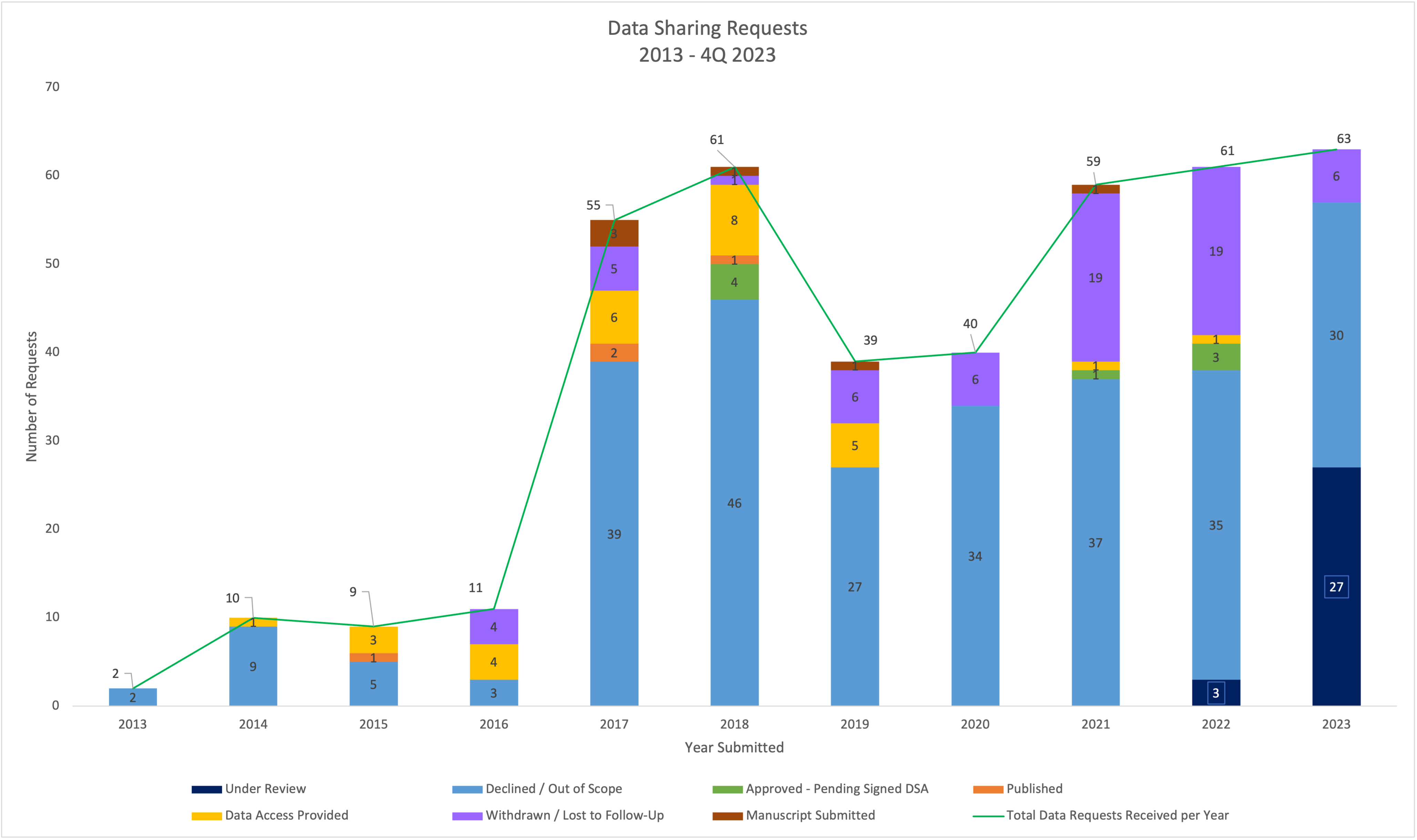
****Requests are logged in these metrics to the year submitted; as a request flows through the process, the updated status is logged to the year that the request was received.
Overall Summary of Requests (Table 1)
| Data Sharing Requests since 2013 | Total |
|---|---|
| Data requests received** | 410 |
| Under review | 30 |
| Declined/Out of scope | 267 |
| Approved - pending signed DSA | 8 |
| Data Access Provided* | 29 |
| Withdrawn / Lost to follow-Up | 66 |
| Published | 4 |
| Manuscript submitted | 6 |
* Also includes 1. requests for summary data and 2. requests for genomics data shared on a public website.
** Includes Proposals and Study Enquiries
*** For declined research proposals and/or study enquiries with more than one reason for a decline, e.g. where more than one study was requested in a single research proposal and/or study enquiries, only one reason for a decline is documented in these metrics.
**** Not a request for secondary analysis by a third-party/independent researcher
Declined/Out of Scope Requests (Table 2)
| Reason for Decline*** | Total |
|---|---|
| < 2 years post database lock | 84 |
| Not a data sharing request**** | 14 |
| Duplicate submission | 10 |
| Not BMS asset or study | 17 |
| Study not in an approved or marketed product | 7 |
| Study completed before 2008 | 4 |
| Study did not pass informed consent check | 38 |
| Final study completion date not reached | 43 |
| Not a phase 2 – 4 Interventional Study | 4 |
| Primary Clinical Trial results have not been published in a peer-reviewed journal | 3 |
| Trial results have not been disclosed in a public registry | 15 |
| The Data Request competes with the BMS publication plan | 28 |
For additional information
For approved requests as of 2021, visit Vivli Approved Research Proposals