Clinical research should gather more than just data. It should find truths. And the knowledge that’s uncovered through this process should be shared so that it can be put into practice more quickly—helping real people in real-world settings. We take that approach at the DCRI.
Comprehensive Capabilities for Confidence at Every Phase
From design to implementation to publication, the DCRI expertly manages all aspects of global clinical trials, from Phases I-IV. DCRI’s experienced operational teams utilize a proactive approach that manages complexity, emphasizes communication, and drives engagement and enrollment. Additionally, we offer deep biostatistical expertise and impeccable data integrity.
Clinical Trial Services Provided by the DCRI Include:
- Duke Early Phase Clinical Research (Phase I)*
- Phase II/III Development
- Device Trials
- Post-Marketing Surveillance/Late-Phase
*Phase I services are coordinated through the DCRI but are provided by the Duke Early Phase Research Unit (DEPRU), part of the Duke School of Medicine.

Worldwide Operational Excellence
The DCRI offers the full-service capabilities of a clinical research organization (CRO) while maintaining the independence of an academic institution. Over 750 operational employees support every phase of clinical development.
Our operational capabilities include:
- Study Design
- Protocol Development
- Regulatory Strategy
- Project Leadership
- Site Management & Monitoring
- Biostatistics & DSMB
- Data Management
- Informatics
- Advanced Biomarkers
- Pharmacometrics
- Imaging Core Lab
- Participant Engagement
- Participant Recruitment / Retention
- Health Economics
- Clinical Events Adjudication
- Safety Surveillance
- Medical Writing
- Publications Management
- Site Contracts & Payments
- Research Communications & Engagement
- Observational Research & Implementation Science
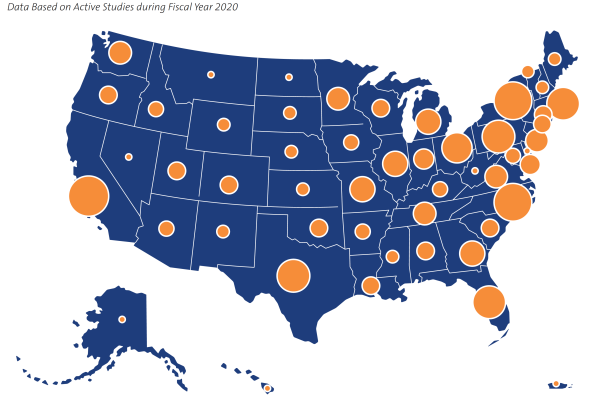
Our Site Network
The depth and breadth of DCRI's collaborations reach around the globe to more than 40 countries. In the U.S. alone, the DCRI site network touches every state plus Puerto Rico, with just over 1,100 sites.