Tackling today’s healthcare challenges requires collaboration from every stakeholder, including patients, academic investigators, site investigators, regulatory agencies, pharmaceutical and device industry leaders, payers, technology companies, and more.
DCRI Think Tanks demonstrate what is possible when you bring smart people together to tackle the biggest challenges in clinical research. From developing innovative approaches to leveraging artificial intelligence, these meetings are about creating pathways to useful, focused discussions across the healthcare continuum and then moving consensus to action.
Our mission: Address the most critical gaps in clinical research by convening leaders across the healthcare industry to map the way forward in designing, conducting, and implementing high-quality, evidence-based research.
Expert Meetings & Resulting Insights
For over 30 years, DCRI Think Tanks have convened leaders across the healthcare industry to map the way forward in designing, conducting, and implementing high-quality, evidence-based research. Below, learn about our most recent meetings and read insights from leading experts, or visit our archive of meetings and publications.
The First and Last Mile of Primary Care
An April 2025 expert panel explored how to transform primary care practices into platforms for clinical research, examining infrastructure needs, innovative trial designs, and collaborative funding models that could unlock the untapped potential of America's healthcare frontline.
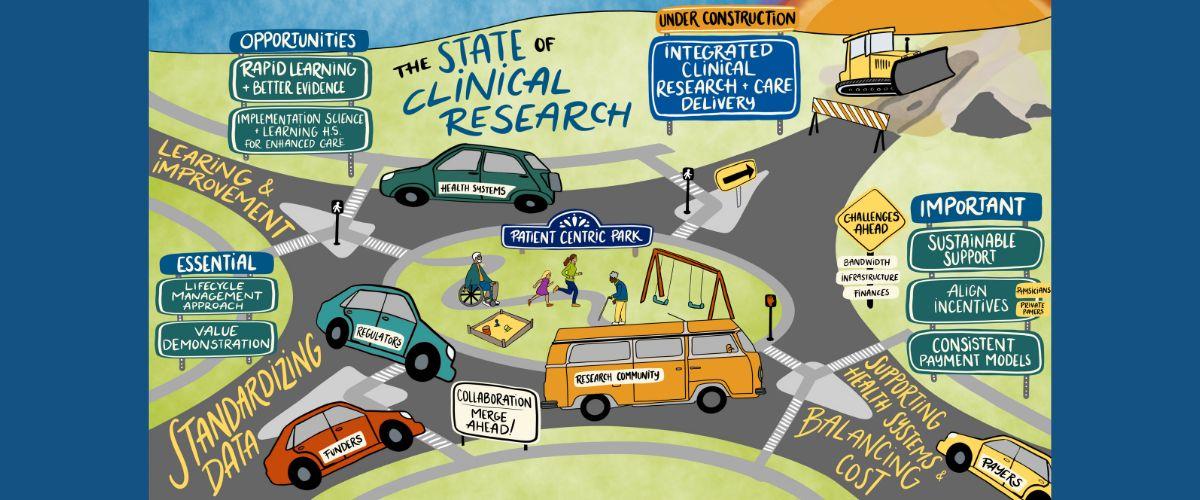
The State of Clinical Research and Healthcare
On July 31, 2024, an expert panel led a DCRI think tank session around recent government initiatives, emerging evidence generation and implementation strategies, and potential changes to payment structures that affect clinical research and care delivery.
Anti-Obesity Pharmacotherapy
The October 2024 DCRI Think Tanks meeting on “Anti-Obesity Pharmacotherapy: An Urgent Need for Guidance and Access” aimed to tackle this chronic condition head-on by bringing together partners from academia, government, industry, and patient/community organizations.
Recent DCRI Think Tanks
From administrative efficiencies to early-stage drug discovery and trial optimization, artificial intelligence (AI) is reshaping the clinical research landscape. The field continues to explore practical applications of AI, including integration into regulatory review, as well as opportunities in late-stage clinical development and post-market surveillance. These expanded applications present new opportunities and challenges that demand thoughtful exploration.
This Think Tank brought together leading experts from across the clinical research ecosystem, including academia, industry, regulatory agencies, patient advocacy, and others, to explore the evolving role of AI in the later stages of clinical development. The program examined how AI has been supporting the execution, conduct, and analysis of the trials, including data collection, transformation, and interpretation. The program also evaluated the standards that have been applied to AI use cases and how they should be developed to inform efficient, transparent, and ethical use of AI within clinical research.
As AI capabilities accelerate, the clinical research field must address the widening gap between technological potential and practical implementation. This program explored how to responsibly advance AI adoption while ensuring safety, compliance, and scientific rigor.
Key Themes and Objectives
- What has AI accomplished in clinical development to date, and what critical gaps remain? What standards have been applied for AI adoption across the clinical research ecosystem?
- How can AI support data collection, transformation, analysis, and interpretation of Phase III and Phase IV FDA-mandated studies?
- How are regulators currently leveraging AI, and what governance, safety, and evidentiary standards are needed?
- How does the FDA’s AI guidance inform algorithm use and risk-based strategies?
- What are the implications of AI-enabled safety data management and post-market surveillance?
Directors: Michael E. Matheny, MD, MS, MPH, FACMI, FAMIA, Vanderbilt, and reekanth Vemulapalli, MD, DCRI; Meeting Fellow: Husam Salah, MBBS, Duke University
As the clinical trial landscape evolves, particularly in the face of increasingly complex and costly outcomes trials, there is growing urgency to identify and validate alternative endpoints that extend beyond mortality and other “hard” outcomes. These alternative endpoints, which may require shorter follow-up and smaller sample sizes, offer the potential to streamline trial design, reduce resource burdens, and accelerate access to effective therapies.
This Think Tank convened a group of stakeholders to explore the opportunities and challenges of advancing surrogate and nontraditional endpoints for regulatory-enabling clinical trials. The discussion drew on cross-disciplinary expertise, with a particular focus on functional measures, surrogate endpoints, and patient-reported outcomes as meaningful indicators of clinical benefit across therapeutic areas.
This session aimed to answer the following key questions:
- Which recent surrogate endpoints gained regulatory approval, and how did disease context affect their acceptance? How quickly did regulators, clinicians, and patients adopt these endpoints?
- In what scenarios were conventional endpoints no longer practical or sufficient to capture meaningful clinical benefit? What factors (regulatory, industry, clinical) perpetuated their continued use?
- How could we expand the use and regulatory acceptability of alternative endpoints, such as imaging, functional measures (e.g., muscle or cognitive function), and patient-centered outcomes (e.g., quality of life or symptom scores)? How did practical use compare in rare versus common diseases and pre- and post-approval?
- What did “known benefit” mean in the context of regulatory certainty? What were the expectations for endpoints that were “reasonably likely to predict clinical benefit”? Did these need to evolve with recent advances in preventative care?
- What special considerations apply when designing trials for prevention or lower-risk populations? How do we take steps towards validating endpoints that inform successful prevention and “efficacy” in lower-risk populations over a manageable timeframe?
Directors: Lesley Inker, MD, MS, Tufts, and Christopher Mosher, MD, MHS, DCRI; Meeting Fellow: Kristin Corey, MD, Duke University
Primary care is the cornerstone of a healthcare system, providing comprehensive and continuous care to patients. It plays a crucial role in managing chronic health conditions, promoting preventive care, and ensuring timely access to medical services. However, due to the volume and breadth of patient care, and limited resources, primary care providers often face significant challenges in integrating clinical research and rapidly implementing research findings into practice.
Meeting attendees discussed these challenges and identified innovative strategies to move the needle on embedding clinical research into primary care practice. Key questions they considered included:
- How is primary care evolving, and where are there opportunities to better integrate clinical research? How do primary care research settings differ from other clinical research sites?
- How can we engage and support primary care clinicians to increase their participation in clinical research? How can we leverage existing primary care research networks?
- What types of clinical trials or other clinical research are most suitable for primary care settings? How do we design trials to fit into primary care settings?
- What role can technology play in supporting primary care clinicians to participate in clinical trials?
- What are the potential use cases of trials in primary care settings that bring value to patients, primary care clinicians and their health systems, and industry partners?
Directors: Rowena Dolor, MD, MHS, DCRI; Russel Rothman, MD, MPP, Vanderbilt; Meeting Fellow: Ryan M. Kane, MD, MPH, Duke University
Given the rapid advancements in science, the pressing health needs, and the drive for greater efficiency, how can we develop platform trials that lead to regulatory-grade analysis in support of registration? Biotechnologies that have the capacity to and are tested for the treatment of multiple diseases will continue to evolve. A single medical product could be crucial for various overlapping populations, which traditionally would have been developed and implemented sequentially. This DCRI Think Tank explored platform trial designs that could span different therapeutic areas and/or leverage precision medicine for simultaneous clinical investigations that lead to regulatory-enabling endpoints to inform registration with regulatory bodies.
Meeting attendees discussed the following themes and opportunities for action:
- In recent years, there has been an explosion in adaptive platform trials, especially for COVID treatments. These trial designs are generally not leveraged for registrational trials – how do we make use more frequent for pivotal trials?
- What are the opportunities and challenges to address for therapies that could be developed for registration using a platform model?
- What are the considerations for platform approaches or scenarios where this would be advantageous for multiple indications, different populations, and at different stages of development?
- What is the value proposition for advancing therapeutics across multiple indications regarding risks, rewards, and cost?
- What are the misaligned incentives across stakeholders that prevent the use of platform trials?
- What does sponsorship look like?
- What are the logistical barriers and challenges with data management?
- What solutions have been utilized to address the logistical barriers in implementing platform designs?
- How can platform designs accelerate early clinical development and support decisions for late-stage development?
Directors: Kanecia Zimmerman, MD, PhD, MPH, DCRI, and Derek Angus, MD, MPH, UPitt; Meeting Fellow: Benjamin Catanese, MD, Duke University
Recent Think Tank Publications
Leaders and Partners
The DCRI Think Tanks Advisory Board

Advisory board partnerships with industry leaders help the DCRI Think Tanks program address the right topics, at the right time, with the right people. Our partners provide crucial insight and connections that go beyond DCRI's clinical and operational expertise. From guidance on attendees, framing discussion topics, and contributions to resulting publications, our advisory board members are players in the lifecycle of every event.