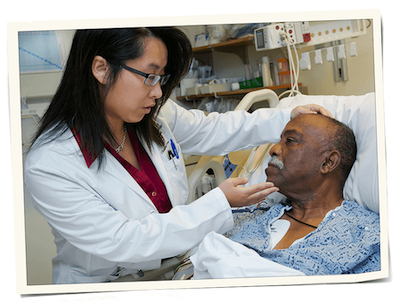
While that work alone is important, just as critical is sharing and implementing the knowledge we gain, as it ensures that our work will have impact patient care.
From publications in top-tier academic journals to presentations at professional conferences, the DCRI has many mechanisms for sharing our new discoveries with the research community.
Our commitment to share knowledge also expands beyond academia, as we deliver recommendations to the FDA, share best practices with clinicians across the U.S., and distill findings into participant-friendly language to share new science with the public.
CHAPTER 1
Bench to Bedside: Closing the Gap Between Knowledge and Practice
Many of DCRI’s faculty are also practicing clinicians—meaning that questions that arise as they care for patients inform their next research projects. This model enables the DCRI to be involved in every step of the research process, from asking the right questions to making sure patients actually benefit from the answers to those questions.
CHAPTER 2
Working With Regulators: How DCRI Shares Knowledge With FDA
The DCRI often shares knowledge gleaned from its research with the FDA. It also participates in public-private partnerships, such as the Cardiac Safety Research Consortium and the Clinical Trials Transformation Initiative, to bring stakeholders together to advance the field.
CHAPTER 3
Changing Care: How Implementation Impacts Outcomes
At the DCRI, rigorous methodology not only enables scientific breakthroughs, but also helps determine best practices for implementing these findings. Through partnering with community providers and studying quality improvement, the DCRI is leading the way in the implementation science space.
Sharing Knowledge to Improve Clinical Practice
- DCRI researchers have published over 16,000 academic publications since 1996, creating a repository of knowledge that has been cited in scientific publications over 760,000 times.
- DCRI faculty are regularly recognized as being in the top 1% of the most highly cited academic researchers In the world.
- Through its unique DCRI Think Tanks program, for over 25 years the DCRI has gathered the brightest minds in the industry from government, academic, and commercial organizations in a non-competitive and collaborative space to discuss and inform policy and practice solutions for patients’ most pressing problems.
- Since 2011, the ARISTOTLE study has generated 83 publications, with nearly 50% of those in high-impact journals—those considered to be highly influential in their field.
- Incorporating nearly 1,000 clinical research sites, the DCRI site network includes every state and Puerto Rico.
DCRI On Record
DCRI-ers past and present take a trip down memory lane to explore the creation of key offerings, favorite memories, and more.
Magnus Ohman, MBBS, one of DCRI's original cardiology faculty, talks with Elizabeth Cook, manager of DCRI's medical communications and scientific publications group, about how her group's work enables the DCRI to better share its scientific discoveries.
Pediatrician Kanecia Zimmerman, MD, MPH, speaks with Patty McAdams and Julia Vail, MA, both communications project managers, about how the DCRI shares knowledge gained from a study with the study's participants and their families.
DCRI has played, and continues to play, a critical leadership role in the medical product development environment—from design and conduct of clinical studies, to infrastructure support of registries, to pressing for cross-disciplinary dialog via its Think Tanks. The latter have done much to shape my outlook on regulatory matters at FDA and to help me see my own role in the ecosystem.
Norman Stockbridge, MD, PhD
Director, Division of Cardiology and Nephrology
Center for Drug Evaluation and Research
U.S. Food and Drug Administration