Hernandez Honored with QCOR Outstanding Lifetime Achievement Award
The American Heart Association's (AHA) Council on Quality of Care and Outcomes Research (QCOR) Council bestowed the Outstanding Lifetime Achievement Award on Adrian Hernandez, MD, MHS, the executive director of the Duke Clinical Research Institute (DCRI) and vice dean at the Duke University School of Medicine, during the AHA Scientific Sessions on November 16. The award is presented annually at the AHA Scientific Sessions to a QCOR Council member for their significant long-term contributions to outcomes research and the improvement of cardiovascular care.
Accelerating Evidence Generation: Addressing Critical Challenges and Charting a Path Forward
Healthcare leaders have united to tackle the mounting challenges of expensive and inefficient drug development in an era of rising healthcare costs and aging populations. Their recommendations, published in the Journal of Clinical and Translational Science, chart a path toward more equitable, efficient, and inclusive clinical trials that better serve diverse communities.
All in the Family
Sisters Rebecca Kirkland and Mary Trent Jones are no strangers to volunteering, spending decades serving on boards, with Rebecca on the Duke University Board of Trustees and the D
DCRI Celebrates 10 Years of Supporting Patient-Centered Research Powered by PCORnet®
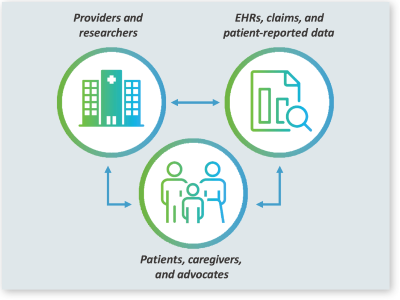
2024 marks the 10-year anniversary of PCORnet® — a national patient-centered clinical research network developed with funding from the Patient Centered Outcomes Research Institute (PCORI) to support a transformational shift toward national-scale, patient-centered comparative clinical effectiveness research. What started as a vision to bypass the traditional silos hindering research insights has since become a powerful infrastructure with proven success across hundreds of studies seeking fast, representative answers that are meaningful to patients and caregivers. The DCRI has played a major role in the success of PCORnet.
New Publication Investigates Practices to Improve Evidence Generation System in Clinical Trials
A new publication developed in collaboration by CTTI, the Duke Clinical Research Institute (DCRI), the Duke Margolis Institute for Health Policy, and Protas published in Trials highlights the current barriers to clinical trial transformation, areas of improvement, and actions needed to transform clinical evidence generation in the United States to better align with clinical care demands. A modern clinical trial infrastructure should prioritize trial accessibility, answer relevant study questions reliably, respond to public health emergencies rapidly, and more.
DCRI Shares Late-Breaking Results, Expert Insights at ESC Congress
Faculty members and fellows of the Duke Clinical Research Institute (DCRI) shared their expertise during the European Society of Cardiology Congress (ESC) in London, Aug. 30-Sept. 2. Late-breaking trial results presented by DCRI faculty at the meeting offered new insights into drug and device efficacy and capabilities.
First Parkinson’s Disease Patient Treated with ALX-001 in Allyx Therapeutics, DCRI Study
Allyx Therapeutics has announced that the first patient has been dosed with its lead compound, ALX-001, in a new clinical study assessing safety, pharmacokinetics and potential therapeutic response in patients with Parkinson’s disease. ALX-001 is a highly selective, first-in-class, synapse-targeted, disease-modifying oral therapy. Allyx Therapeutics is a clinical-stage biotechnology company working to deliver a novel approach to preserve and protect synapses for people living with neurodegenerative diseases.
Wearable Heart Monitor Increases Diagnosis of Irregular Heart Rhythm
Wearable, long-term continuous heart monitors helped identify 52% more cases of atrial fibrillation compared to usual care, but that did not lead to a reduction in hospitalizations due to stroke, according to a study led by the Duke Clinical Research Institute.
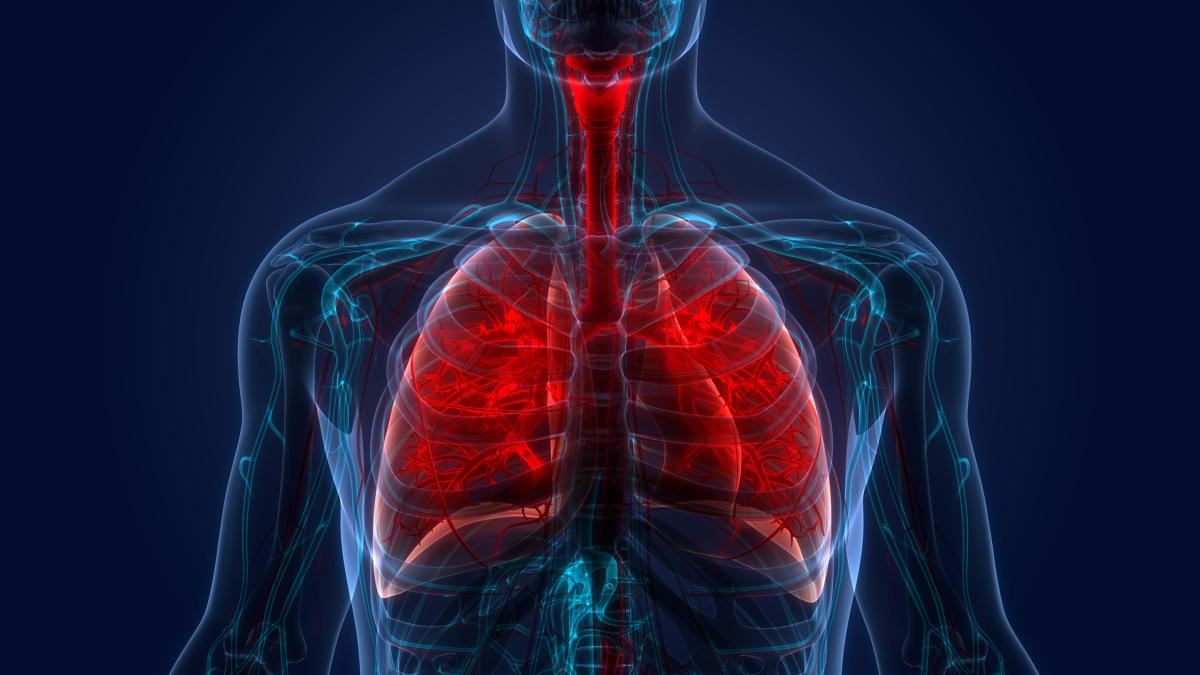
DCRI Unveils New Imaging and Translational Biomarker Evidence in Pulmonary Fibrosis and Lung Disease from the IPF-PRO/ILD-PRO Registry
New evidence and results from eight studies based on data from the IPF-PRO/ILD-PRO Registry was shared by the Duke Clinical Research Institute (DCRI) and its collaborators at the American Tho
3 DCRI Faculty Receive Duke School of Medicine Awards
Three Duke Clinical Research Institute faculty members were honored by the Duke School of Medicine during the school’s annual faculty awards ceremony on Tuesday, May 14.