Clinical Events Classification (CEC), Safety Surveillance (SS), Imaging Core Lab (ICL), Arrhythmia Core Lab (ACL), Hemodynamics Core Lab (HCL)

DCRI CSI+ provides a cross-functional approach to core trial medical requirements, ensuring comprehensive, independent, and efficient assessment of efficacy and safety. Our services include event adjudication, safety surveillance, physician-led clinical study site support for protocol and enrollment inquiries, enrollment validation, and centralized imaging core labs for electrophysiology and multi-modal imaging interpretation.
Our collaborative approach across trial partners ensures streamlined systems and operations to systematically generate validated, high-quality data. By leveraging CSI+ clinical thought leadership, a specialized healthcare provider operations team, and deep technical expertise, we deliver robust and reliable results that meet the highest standards of scientific rigor and regulatory compliance.
CSI+ Trial Support Services
The DCRI Clinical Events Classification (CEC) group uses innovative strategies, including streamlined data workflows, so that adjudicated endpoint data are available on time for the Independent Data Monitoring Committee (IDMC), database lock, and other important timelines throughout a trial. CEC activities are essential to secure FDA or EMA approval for drugs and devices in a wide range of therapeutic areas.
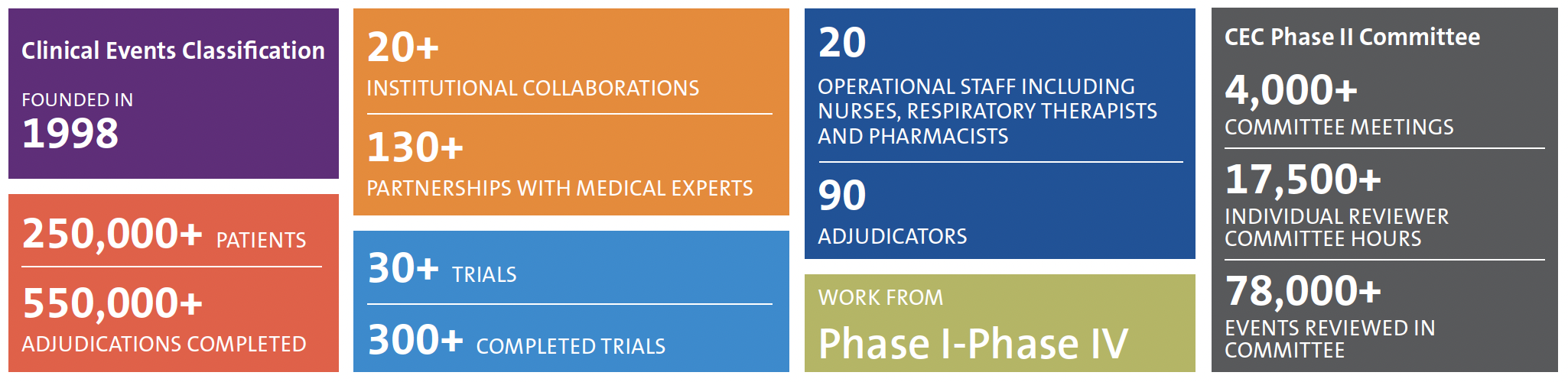
CEC Solutions
CEC has experience in adjudication across numerous therapeutic areas, including cardiovascular studies, respiratory medicine, infectious diseases, gastrointestinal and liver disease, kidney disease, and pediatrics. Comprehensive activities for the development of highly efficient events adjudication programs include:
- A tailored approach to efficacy review and event validation that is adaptable to protocol requirements in both model and cost.
- Clinical events adjudication processes, performed in 21 Code of Federal Regulations (CFR) part 11 compliant adjudication systems, that enable secure, global access for all physician reviewers.
- Collaboration in protocol development. Clinical preparation and review of event packets dossiers within a secure adjudication system.
- Proven leadership in providing adjudication guidance aligned with regulatory agencies.
- Leadership in peer-reviewed publications on clinical events, adjudication processes, and results.
- Robust quality control processes that ensure accurate data.
- Access to an international group of experts, including faculty thought leaders and CEC adjudicators experienced in clinical trials and event review/adjudication in many therapeutic areas.
- Systematic, comprehensive, unbiased, blinded, and independent clinical event adjudication of suspected events with emphasis on quality and event turnaround times.
The DCRI CEC team provides expert adjudication activities that are unique and customizable to each trial. Discover the specialized approach that sets us apart:
- The DCRI established one of the first event adjudication groups worldwide to perform independent and unbiased event adjudication as a key element in providing trial endpoint data.
- Operational excellence with a core CEC team that has extensive event adjudication and clinical experience.
- A successful history leading event adjudication activities and promoting confidence in the management and processing of events/cases.
- Trial tested processes and systems ensure project timelines are met with the highest level of quality and rigor expected by regulatory agencies, including adjudication process development in collaboration with the FDA.
- Input on clinical endpoint definitions, eCRF collection of key endpoint data, and collaboration with Safety Surveillance on negatively adjudicated events assures consistency with clinical practice and regulatory expectations and minimizes the burden of source document collection.
Take the CEC Fact Sheet With You
The Backbone of Our Work
CEC’s work affects patient lives and is one of the most critical steps in the clinical research process to ensure accurate clinical outcomes. Each project is met with a commitment to:
Efficiency
- Rigor and discipline in every project
- Streamlining communications and seamlessly managing the adjudication process
- Continuing the proven track record of meeting or exceeding milestones
Accuracy
- An obsession with quality
- The drive to get it done right the first time to avoid double work
- Extensive experience submitting data to the FDA and EMA, with firsthand knowledge of the standards they expect
Innovation
- Adjudication algorithms/auto adjudication processes to programmatically define adjudication values and event type details
- Leadership in future advancements in adjudication, including the use of electronic medical records and machine learning to streamline adjudication of clinical events
- Implementation of complex trigger programming to support a robust and efficient event identification process
Learn More About DCRI Clinical Events Classification
DCRI Safety Surveillance provides on-time ascertainment, clinical evaluation, and reporting of serious adverse events (SAEs), adverse device effects (ADEs), and other safety events through protocol-aligned safety management processes.
In partnership with clinical nursing and pharmacy backgrounds, Duke/DCRI faculty safety medical monitors and expert safety surveillance associates employ best-in-class medical, regulatory, and data support to ensure reliable, timely, and accurate safety processes and systems customized for every project. Across study phases, intervention types, and therapeutic areas, DCRI Safety Surveillance adapts to each protocol’s unique risks, requirements, and safety event data flow to ensure timely capture, assessment, and reporting of SAEs and other safety events for a study’s sites and participants, globally.
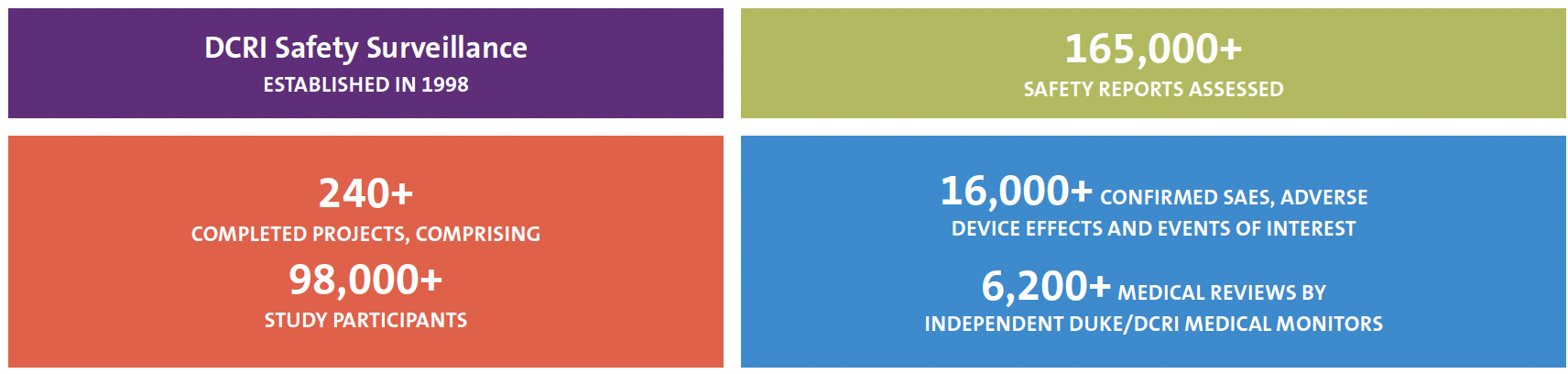
Customized and Compliant Safety Solutions
DCRI Safety Surveillance is committed to quality, compliance, and patient safety. Our approach is customized to each sponsor’s needs and optimal cost effectiveness:
- Provide specifications and user testing for all EDC forms relevant to safety event reporting; for the project-specific safety database, and on-demand reports
- With DCRI data security and regulatory experts, establish safety event management from DCRI’s first awareness to the final report to the sponsor
- Develop and implement protocol-aligned Safety Management Plan with training, instruction, and support across DCRI Safety Associates, all site investigators, and global staff, ensuring consistent safety event processing and reporting
- Processes for rapid safety event discovery and assessment, whether captured via EDC, provider contact, study participant portal or app, or call center logs of healthcare encounters
- Unbiased, blinded medical review of events with an assessment of expedited reporting criteria by DCRI Safety Medical Monitors
- Generation of MedWatch/CIOMs reports for regulatory reporting and site/IRB notification
- DSMB support on request with SAE/safety event reports and presentation
- Safety event reconciliation of critical variables between clinical and safety databases at intervals and pre-database lock
- Analyses of Similar Events, medical review of aggregate AE/SAE listings, safety surveillance/pharmacovigilance research collaborations
Keep the Safety Surveillance Fact Sheet on Hand
DCRI Safety Medical Monitors
Our safety medical monitors include DCRI and Duke faculty thought leaders and clinician-researchers, providing timely, blinded, unbiased medical review of each safety event reported. They are experts in assessing safety for individual patients, trials of varying sizes, and clinical programs.
Learn more about DCRI Safety Surveillance
The DCRI Imaging Core Lab is at the forefront of imaging clinical research, with Duke Imaging and Radiology faculty and staff authoring national standards for clinical care and research. The program is one of the most respected imaging clinical trials groups in the world, providing experienced oversight and independent imaging management for all phases of clinical trials, including multicenter industry trials.

World Class Technology and Innovative Leadership
The DCRI’s Imaging Core Lab continually builds on its record of success by continually adding new technologies and cutting-edge capabilities. Our imaging expertise encompasses all modalities and applications:
- Computed tomography
- Coronary Computed Tomography Angiography
- Coronary Artery Calcium Scans
- Echocardiography (2D, 3D, Stress, Strain, TEE)
- Electrocardiography
- AI Applications
- Hemodynamics
- Magnetic resonance imaging
- Ophthalmology: CT/KT, FP, ОСТ
- Positron emission tomography
- Vascular Ultrasound
- X-ray
- Angiography
DCRI Imaging Offerings
Thought Leadership
- Trial design
- Customized imaging protocol
- State-of-the-art imaging techniques
- Results interpretation and dissemination
Efficient and knowledgeable operations
- Project management
- Trial setup
- Site training
- Site image acquisition and analysis
- Reproducibility testing and remediation
- Data quality assurance
- Compliant with good clinical practice guidelines
Image-management solutions
- Web-based image transfer linked to EDC
- Web-based PACs system for site and sponsor review
- Secure, cloud-based image storage
- Compliant to 21CFR Part 511
- State-of-the-art analysis systems
Regulatory and policy expertise
- FDA panels and committees
- Submission experience
- National guidelines authorship
View the Imaging Core Lab Fact Sheet
Learn More about DCRI Imaging
DCRI’s Arrhythmia Core Laboratory (ACL) provides high-quality evaluation, adjudication, and validation of electrocardiographic and electrogram review. The ACL has extensive experience in heart rhythm monitoring,12-lead electrocardiogram and device-based electrogram event adjudication, arrhythmia science, clinical trial design and execution, and outcomes research.
The DCRI ACL has one of the largest groups of Heart Rhythm Society board-certified clinical cardiac electrophysiology adjudicators in the world. We have enrolled more patients in heart rhythm-related trials than any other academic research organization.
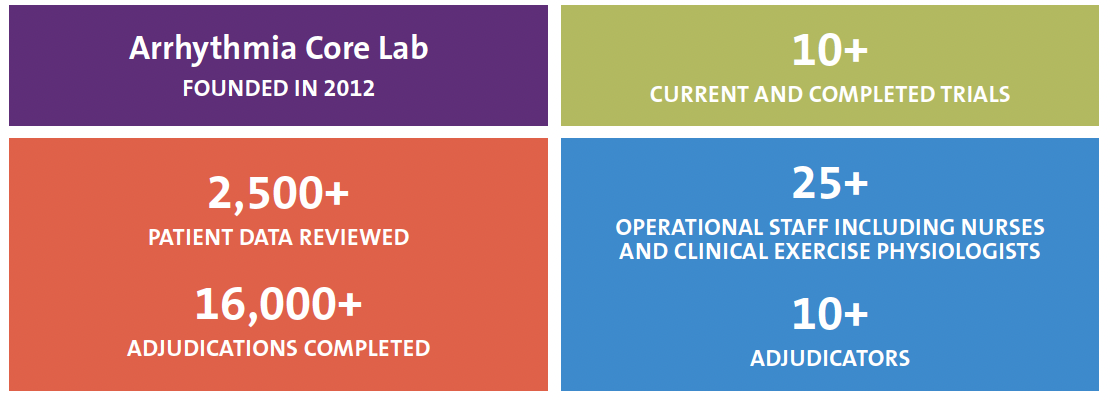
Capabilities and Expertise
The ACL provides heart rhythm evaluation and adjudication across several modalities and multiple study designs and settings. Our services include:
- Evaluation and adjudication of 12-lead electrocardiograms
- Evaluation and adjudication of ambulatory monitoring tracings and results
- Evaluation and adjudication of device-based diagnostics and electrograms (including but not limited to pacemaker, implantable cardioverter defibrillator, and cardiac resynchronization therapy recordings)
- Design and implementation of core laboratory protocols, study manuals, and procedures
- Quality assurance evaluations and validation for the blinded adjudication process
- Publication and dissemination of study results
- Pre-clinical, phase I, II, and III clinical trials, post-marketing, and observational studies
- Clinical trial design, support, and execution
In addition, our electronic adjudication system technology allows reviewers to adjudicate electronically with an Internet connection; track the workflow process, providing a full audit trail (21 CFR part 11-compliant; produce reports from executive summary to detailed information; and provide electronic dossiers.
Take the Arrhythmia Core Lab's Fact Sheet With You
Learn More about the DCRI Arrhythmia Core Lab

The DCRI Hemodynamics Core Lab (HCL) leverages an expert group of more than 15 practicing physicians providing independent adjudication of hemodynamic tracings from right and left heart catheterizations for drug and device trials. With high consistency and efficiency, we offer both blinded and unblinded adjudication, establishing the gold-standard in hemodynamic adjudication.
Hemodynamics tracings at DCRI are independently adjudicated by a group of expert practicing cardiologists who are actively treating patients. Our collaborative approach:
- Efficient readings, rapid results
- Cost-effective analysis
- Counter-reading by multiple readers for cross-validation, ensuring consistency and accuracy
- Broad bandwidth to serve multiple large and small-scale studies
- Expert data quality assurance, analysis, and retention
- Flexible workstreams to support study-specific requirements
- Meets the FDA’s rigorous requirements for validation and compliance
























